Advertisements
Advertisements
Question
Show how will you synthesize pentan-1-ol using a suitable alkyl halide.
Solution
Hydrolysis of 1-bromopentane by aqueous NaOH gives pentan-1-ol.
\[\ce{\underset{1-bromopentane}{CH3 - CH2 - CH2 - CH2 - CH2 - Br} + NaOH ->[\Delta][S_{N}2 Hydrolysis ] \underset{pentan-1-ol}{CH3CH2CH2CH2 - CH2 - OH} + NaBr}\]
APPEARS IN
RELATED QUESTIONS
Write the final product(s) in each of the following reactions:
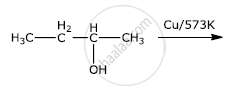
Write the mechanism of the following reaction:
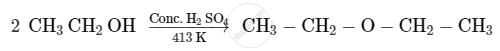
Give the equation of the following reaction:
Treating phenol with chloroform in the presence of aqueous NaOH.
Name the reagent used in the following reaction:
Oxidation of a primary alcohol to carboxylic acid.
Name the reagent used in the following reaction:
Benzyl alcohol to benzoic acid.
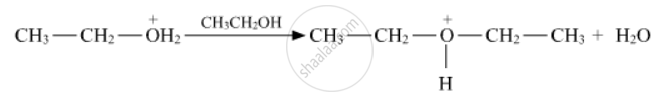
Write the mechanism (using curved arrow notation) of the following reaction :
Lucas reagent is ____________.
Lucas test is used for the detection of _____________.
In the reduction \[\ce{R - CHO + H2 -> RCH2OH}\] the catalyst used is:
Lucas test is done to differentiate between ____________.
Which one of the following on oxidation gives a ketone?
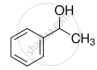
Identify the secondary alcohols from the following set:
- \[\ce{CH3CH2CH(OH)CH3}\]
- \[\ce{(C2H5)3COH}\]
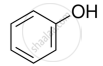
Name the factors responsible for the solubility of alcohols in water.
Alcohols react with active metals e.g. Na, K etc. to give corresponding alkoxides. Write down the decreasing order of reactivity of sodium metal towards primary, secondary and tertiary alcohols.
Explain why is OH group in phenols more strongly held as compared to OH group in alcohols.
Ethers can be prepared by Williamson synthesis in which an alkyl halide is reacted with sodium alkoxide. Di-tert-butyl ether can’t be prepared by this method. Explain.
The correct geometry around oxygen in CH3OCH3 is
Which of the following alcohols will not undergo oxidation?
Which of the following observation is shown by 2-phenyl ethanol with Lucas Reagent?
Write the mechanism of acid dehydration of ethanol to yield ethene.
