Advertisements
Advertisements
Question
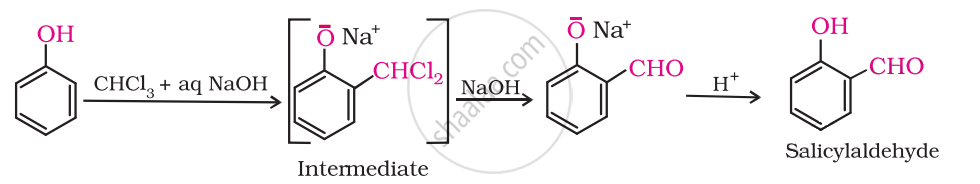
Give the equation of the following reaction:
Treating phenol with chloroform in the presence of aqueous NaOH.
Solution
APPEARS IN
RELATED QUESTIONS
Write the final product(s) in each of the following reactions:
Ortho and para nitrophenols are more acidic than phenol. Draw the resonance structures of the corresponding phenoxide ions.
Write the mechanism of acid-catalysed dehydration of ethanol to yield ethene.
Name the reagent used in the following reaction:
Oxidation of a primary alcohol to carboxylic acid.
Name the reagent used in the following reaction:
Oxidation of a primary alcohol to aldehyde.
Lucas reagent is ____________.
The compound which reacts fastest with Lucas reagent at room temperature is:
In the reduction \[\ce{R - CHO + H2 -> RCH2OH}\] the catalyst used is:
By which of the following methods alcohol can be prepared in excellent yield?
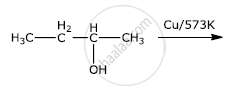
Which of the following is not true in case of reaction with heated copper at 300°C?
Primary and secondary alcohols on the action of reduced copper give:
Mark the correct increasing order of reactivity of the following compounds with HBr/HCl.
| (a) | 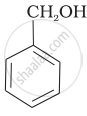 |
| (b) | 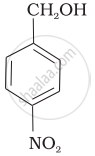 |
| (c) | 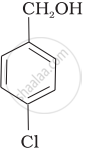 |
Alcohols react with active metals e.g. Na, K etc. to give corresponding alkoxides. Write down the decreasing order of reactivity of sodium metal towards primary, secondary and tertiary alcohols.
What is Lucas reagent?
The correct geometry around oxygen in CH3OCH3 is
Write the mechanism of acid dehydration of ethanol to yield ethene.
Write the mechanism of acid dehydration of ethanol to yield ethene.
Write the mechanism of acid dehydration of ethanol to yield ethene.
Write the mechanism of acid dehydration of ethanol to yield ethene.
