Advertisements
Advertisements
Question
Name the reagent used in the following reaction:
Oxidation of a primary alcohol to aldehyde.
Solution
Pyridinium chlorochromate (PCC), C5H5NH+Cr CrO3Cl− (in CH2Cl2) or pyridinium dichromate (PDC), \[\ce{(C5H5N^+H)2Cr2O^-_7}\] (in CH2Cl2) is used in the oxidation of a primary alcohol to aldehyde.
APPEARS IN
RELATED QUESTIONS
Give reasons for the following:
o-nitrophenol is more acidic than o-methoxyphenol.
Write the mechanism of the following reaction :
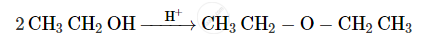
Show how will you synthesize pentan-1-ol using a suitable alkyl halide.
Give the equation of the following reaction:
Oxidation of propan-1-ol with alkaline KMnO4 solution.
Write the mechanism (using curved arrow notation) of the following reaction :
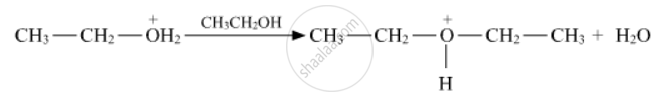
Lucas reagent is ____________.
Lucas test is used for the detection of _____________.
Which of the following are used to convert RCHO into RCH2OH?
(i) H2/Pd
(ii) LiAlH4
(iii) NaBH4
(iv) Reaction with RMgX followed by hydrolysis
Lucas test is done to differentiate between ____________.
Primary and secondary alcohols on the action of reduced copper give:
During dehydration of alcohols to alkenes by heating with cone. H2SO4 the initial step is ____________.
Alcohols react with active metals e.g. Na, K etc. to give corresponding alkoxides. Write down the decreasing order of reactivity of sodium metal towards primary, secondary and tertiary alcohols.
Explain why nucleophilic substitution reactions are not very common in phenols.
In Kolbe’s reaction, instead of phenol, phenoxide ion is treated with carbon dioxide. Why?
What is Lucas reagent?
Which of the following observation is shown by 2-phenyl ethanol with Lucas Reagent?
Write the mechanism of acid dehydration of ethanol to yield ethene.
Write the mechanism of acid dehydration of ethanol to yield ethene.
