Advertisements
Advertisements
Question
Mark the correct increasing order of reactivity of the following compounds with HBr/HCl.
| (a) | 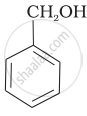 |
| (b) | 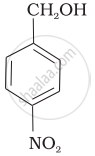 |
| (c) | 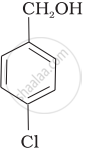 |
Options
a < b < c
b < a < c
b < c < a
c < b < a
Solution
b < c < a
Explanation:
It is type of nucleophilic substitution reaction followed by SN1 mechanism. SN1 mechanism depends on the stability of carbonation. Presence of electron-withdrawing group will decrease the stability of carbocation.
APPEARS IN
RELATED QUESTIONS
Show how will you synthesize pentan-1-ol using a suitable alkyl halide.
Lucas test is used for the detection of _____________.
In the reduction \[\ce{R - CHO + H2 -> RCH2OH}\] the catalyst used is:
By which of the following methods alcohol can be prepared in excellent yield?
Which of the following is not true in case of reaction with heated copper at 300°C?
Lucas test is done to differentiate between ____________.
Which one of the following on oxidation gives a ketone?
Suggest a reagent for the following conversion.

In Kolbe’s reaction, instead of phenol, phenoxide ion is treated with carbon dioxide. Why?
Write the mechanism of acid dehydration of ethanol to yield ethene.
