Advertisements
Advertisements
Question
Ortho and para nitrophenols are more acidic than phenol. Draw the resonance structures of the corresponding phenoxide ions.
Solution
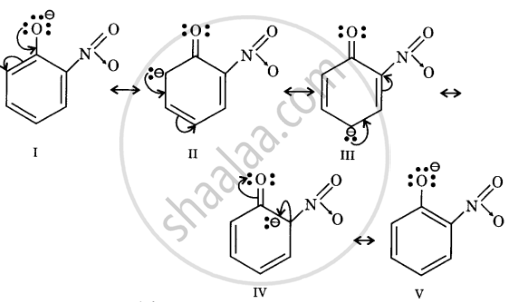
Resonance structures of o-nitrophenoxide ion
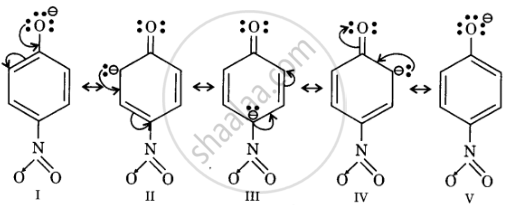
Resonance structures of p-nitrophenoxide ion
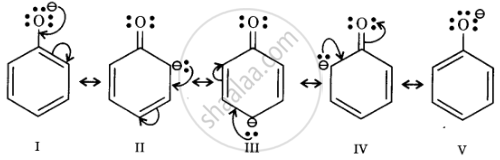
Resonance structures of phenoxide ion
In substituted phenols, electron withdrawing groups such as nitro group increase the acidic strength of phenol. This effect becomes more potent when such groups are present at ortho and para positions. This is because of the effective delocalisation of the anion of the phenoxide ion. Hence, o- and p-nitrophenols are more acidic than phenol.
APPEARS IN
RELATED QUESTIONS
Write the final product(s) in each of the following reactions:
Write the mechanism of the following reaction:
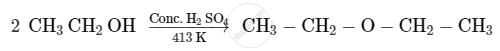
Write the mechanism of the following reaction :
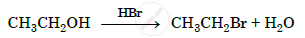
Show how will you synthesize pentan-1-ol using a suitable alkyl halide.
Give the equation of the following reaction:
Oxidation of propan-1-ol with alkaline KMnO4 solution.
Give the equation of the following reaction:
Treating phenol with chloroform in the presence of aqueous NaOH.
Name the reagent used in the following reaction:
Oxidation of a primary alcohol to aldehyde.
The compound which reacts fastest with Lucas reagent at room temperature is:
In the reduction \[\ce{R - CHO + H2 -> RCH2OH}\] the catalyst used is:
By which of the following methods alcohol can be prepared in excellent yield?
Which of the following is not true in case of reaction with heated copper at 300°C?
During dehydration of alcohols to alkenes by heating with cone. H2SO4 the initial step is ____________.
Name the factors responsible for the solubility of alcohols in water.
Suggest a reagent for the following conversion.

The correct geometry around oxygen in CH3OCH3 is
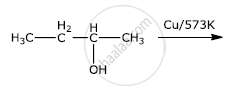
What happens when (CH3)3 C – OH is heated with Cu/573 K?
Write the chemical equation in support of your answer.
Write the mechanism of acid-catalysed dehydration of ethanol to yield ethene.
Write the mechanism of acid dehydration of ethanol to yield ethene.
Write the mechanism of acid dehydration of ethanol to yield ethene.
