Advertisements
Advertisements
Question
Predict the major product of acid catalysed dehydration of butan-1-ol.
Solution
Acid catalysed dehydration of butan-1-ol produces but-2-ene as the main product and butan-1-ene as the secondary product. This is because dehydration of alcohols takes place through carbocation intermediates. Again, butan-1-ol being a 1° alcohol, on protonation and elimination of H2O, first gives a 1° carbocation (I), which being less stable rearranges to form more stable 2° carbocation (II), then it removes a proton in two different ways to form butan-2-ene or butan-1-ene. Since butan-2-ene is more stable, it is the main product according to Setzeff rule.

APPEARS IN
RELATED QUESTIONS
Write the main product(s) in each of the following reactions:
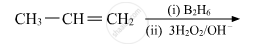
Write the structures of the main products when acetone (CH3 − CO − CH3) reacts with the following reagents :
CH3MgBr and then H3O+
Predict the major product of acid catalysed dehydration of 1-methylcyclohexanol.
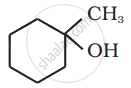
Show how you would synthesise the following alcohol from an appropriate alkene?
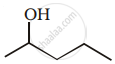
Show how you would synthesise the following alcohol from an appropriate alkene?
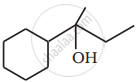
Write the structures of A, B and C in the following reactions :
Ketones react with Grignard reagent to produce ____________.
Aldehydes are reduced to the corresponding alcohols by the addition of hydrogen in the presence of catalysts to form ____________.
Identify ‘C’ in the following:
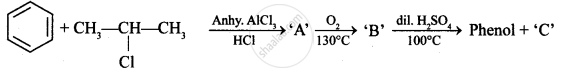
Select the acid(s) which cannot be prepared by Grignard reagent.
Glycerol is used in the manufacture
Alkaline hydrolysis of an alkyl halide can be preferably carried out using ______.
The best reagent to convert pent - 3 - en - 2 - ol into pent - 3 - en - 2 one is ______.
The major product of acid catalysed dehydration of 1-methylcyclohexanol is ______.
For distinction between \[\ce{CH3CHO}\] and \[\ce{C6H5CHO}\] the reagent used is ______.
The major product of the following reaction is:
\[\begin{array}{cc}
\ce{Cl}\phantom{.........................}\\
|\phantom{..........................}\\
\ce{CH3 - CH - CH3 ->[(i) Alc. KOH][(ii) HBr/peroxide (iii) aq. KOH]}
\end{array}\]
Given below are two statements:
Statement I: On heating with KHSO4, glycerol is dehydrated and acrolein is formed.
Statement II: Acrolein has a fruity odour and can be used to test glycerol's presence.
Choose the correct option.
