Advertisements
Advertisements
Question
The major product of acid catalysed dehydration of 1-methylcyclohexanol is ______.
Options
1-methylcyclohexane
1-methylcyclohexene
1-cyclohexylmethanol
1-methylenecyclohexane
Solution
The major product of acid catalysed dehydration of 1-methylcyclohexanol is 1-methylcyclohexene.
Explanation:
According to Saytzeff's rule, i.e, highly substituted alkene is major product. Here dehydration reaction takes place, the alkene is formed due to the removal of a water molecule.
APPEARS IN
RELATED QUESTIONS
Write the main product(s) in each of the following reactions:
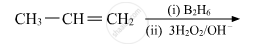
What is meant by hydroboration-oxidation reaction? Illustrate it with an example.
How is the following conversion carried out?
\[\ce{Ethyl magnesium chloride -> Propan-1-ol}\]
Name the reagent used in the following reaction:
Dehydration of propan-2-ol to propene.
Write the structures of A, B and C in the following reactions :
Benzaldehyde differs from acetaldehyde in that:
The reagent used for dehydration of an alcohol is
When alcohol react with concentrated H2SO4 intermediate compound formed is
An aldehyde isomeric with allyl alcohol gives phenyl hydrazone. Pick out a ketone that too gives a phenyl hydrazone containing the same percentage of nitrogen.
The major product of the following reaction is:
\[\begin{array}{cc}
\ce{Cl}\phantom{.........................}\\
|\phantom{..........................}\\
\ce{CH3 - CH - CH3 ->[(i) Alc. KOH][(ii) HBr/peroxide (iii) aq. KOH]}
\end{array}\]
