Advertisements
Advertisements
Question
How is the following conversion carried out?
\[\ce{Ethyl magnesium chloride -> Propan-1-ol}\]
Solution
When ethyl magnesium chloride is treated with methanal, an adduct produces propan-1-ol on hydrolysis.
\[\begin{array}{cc}
\ce{H}\phantom{.................................}\\
\backslash\phantom{..............................}\\
\ce{C = O + C2H5 - MgCl ->}\phantom{.}\\
/\phantom{..............................}\\
\ce{H}\phantom{.................................}
\end{array}\]\[\begin{bmatrix}
\ce{CH2 - \overset{-}{O}\overset{+}{M}gCl}\\
|\phantom{.............}\\
\ce{C2H5}\phantom{.........}\\
\ce{Adduct}
\end{bmatrix}\]\[\begin{array}{cc}
\ce{->[H2O]}
\ce{\underset{Propan-1-ol}{C3H7 - OH} + Mg(OH)Cl}
\end{array}\]
APPEARS IN
RELATED QUESTIONS
Show how is the following alcohol prepared by the reaction of a suitable Grignard reagent on methanal?
\[\begin{array}{cc}
\ce{CH3 - CH - CH2OH}\\
|\phantom{......}\\
\ce{CH3}\phantom{...}
\end{array}\]
Predict the major product of acid catalysed dehydration of 1-methylcyclohexanol.
What is meant by hydroboration-oxidation reaction? Illustrate it with an example.
Name the reagent used in the following reaction:
Dehydration of propan-2-ol to propene.
Name the reagent used in the following reaction:
Butan-2-one to butan-2-ol.
Write the structures of A, B and C in the following reactions :
Ketones react with Grignard reagent to produce ____________.
Acetone reacts with Grignard reagent to form:
Identify ‘C’ in the following:
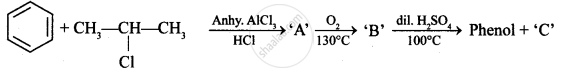
Why is the reactivity of all the three classes of alcohols with conc. \[\ce{HCl}\] and \[\ce{ZnCl2}\] (Lucas reagent) different?
The Wittig reaction is a reaction between a carbonyl compound (aldehyde or ketone only) and a species known as a phosphoniumylide. What is the expected final product in the Wittig reaction?
Most readily hydrolysed halide is:-
When alcohol react with concentrated H2SO4 intermediate compound formed is
The best reagent to convert pent - 3 - en - 2 - ol into pent - 3 - en - 2 one is ______.
An aldehyde isomeric with allyl alcohol gives phenyl hydrazone. Pick out a ketone that too gives a phenyl hydrazone containing the same percentage of nitrogen.
\[\ce{C3H8O ->[{[O]}][K2Cr2O7/H2SO4] C3H6O ->[I2 + NaOH(aq.)] CHI3}\]
In this reaction the first compound is:
For distinction between \[\ce{CH3CHO}\] and \[\ce{C6H5CHO}\] the reagent used is ______.
Given below are two statements:
Statement I: On heating with KHSO4, glycerol is dehydrated and acrolein is formed.
Statement II: Acrolein has a fruity odour and can be used to test glycerol's presence.
Choose the correct option.
Ceric aminonium nitrate and CHCl3/alc. KOH are used for the identification of functional groups present in ______ and ______ respectively.
