Advertisements
Advertisements
Question
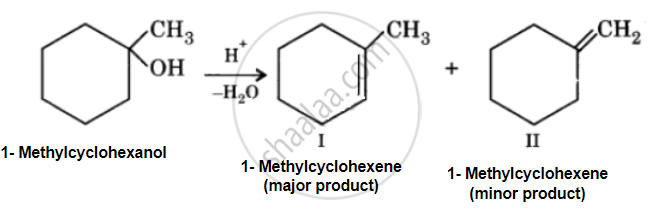
Predict the major product of acid catalysed dehydration of 1-methylcyclohexanol.
Solution
Acid catalyzed dehydration of 1-methylcyclohexanol can give two products, I and II. Since product (I) is more highly substituted, it is the major product according to the Setzeff rule.
APPEARS IN
RELATED QUESTIONS
Name the reagents used in the following reactions:

How is the following conversion carried out?
\[\ce{Propene -> Propan-2-ol}\]
How is the following conversion carried out?
\[\ce{Methyl magnesium bromide → 2-Methylpropan-2-ol}\]
Name the reagent used in the following reaction:
Butan-2-one to butan-2-ol.
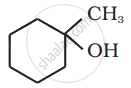
Show how you would synthesise the following alcohol from an appropriate alkene?
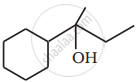
Primary alcohols are prepared by the reduction of carboxylic acids. Though lithium aluminium hydride is a strong reducing agent, it is not used in the reaction. This is so because:
Ethyl alcohol can be prepared from Grignard reagent by the reaction of ____________.
Which of the following reacts with NaOH to give alcohol?
Identify ‘C’ in the following:
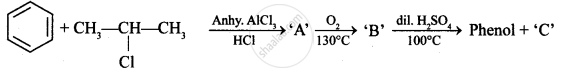
Explain a process in which a biocatalyst is used in industrial preparation of a compound known to you.
The Wittig reaction is a reaction between a carbonyl compound (aldehyde or ketone only) and a species known as a phosphoniumylide. What is the expected final product in the Wittig reaction?
Carboxylic acids are more acidic than phenol and alcohol because of
The best reagent to convert pent - 3 - en - 2 - ol into pent - 3 - en - 2 one is ______.

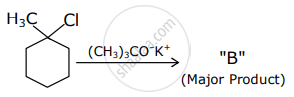
The products "A" and "B" formed in above reactions are:
An aldehyde isomeric with allyl alcohol gives phenyl hydrazone. Pick out a ketone that too gives a phenyl hydrazone containing the same percentage of nitrogen.
For distinction between \[\ce{CH3CHO}\] and \[\ce{C6H5CHO}\] the reagent used is ______.
\[\ce{? ->[\Delta, CN-][EtOH, H2O]}\] Benzoin.
The reactant is obtained by dry distillation of the calcium salts of the following pairs:
Given below are two statements:
Statement I: On heating with KHSO4, glycerol is dehydrated and acrolein is formed.
Statement II: Acrolein has a fruity odour and can be used to test glycerol's presence.
Choose the correct option.
