Advertisements
Advertisements
Question
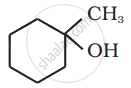
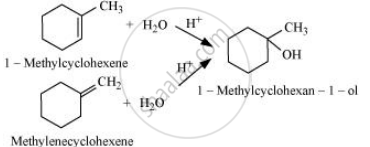
Solution
The given alcohols can be synthesized by applying Markovnikov’s rule of acid-catalyzed hydration of appropriate alkenes.
APPEARS IN
RELATED QUESTIONS
Name the reagents used in the following reactions:

Predict the major product of acid catalysed dehydration of 1-methylcyclohexanol.
Write the mechanism of hydration of ethene to yield ethanol.
How is the following conversion carried out?
\[\ce{Propene -> Propan-2-ol}\]
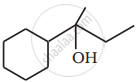
Show how you would synthesise the following alcohol from an appropriate alkene?
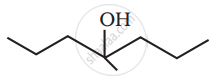
Show how you would synthesise the following alcohol from an appropriate alkene?
Name the reagents used in the following reactions:

Ethyl alcohol can be prepared from Grignard reagent by the reaction of ____________.
Commercially carboxylic acids are reduced to alcohols by converting them to the ______.
Identify ‘C’ in the following:
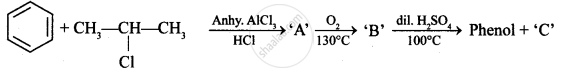
How can propan-2-one be converted into tert- butyl alcohol?
Explain a process in which a biocatalyst is used in industrial preparation of a compound known to you.
Select the acid(s) which cannot be prepared by Grignard reagent.
The reagent used for dehydration of an alcohol is
Glycerol as a trimester present in
When glycol is heated with dicorboxylic acid the product are
To synthesise 1.0 mole of 2-methylpropan-2-ol from Ethylethanoate ______ equivalents of CH3MgBr reagent will be required. (Integer value)
How are the following conversion carried out?
\[\ce{Methyl magnesium bromide -> 2-Methylpropan-2-ol}\]
