Advertisements
Advertisements
Question
Explain a process in which a biocatalyst is used in industrial preparation of a compound known to you.
Solution
Ethanol, \[\ce{C2H5OH}\], is obtained commercially by fermentation, the oldest method is from sugars. The sugar in molasses, sugarcane or fruits such as grapes is converted to glucose and fructose, (both of which have the formula \[\ce{C6H12O6}\]), in the presence of an enzyme, invertase. Glucose and fructose undergo fermentation in the presence of another enzyme, zymase, which is found in yeast.
\[\ce{C12H22O11 + H2O ->[Invertase] \underset{Glucose}{C6H12O6} + \underset{Fructose}{C6H12O6}}\]
\[\ce{C6H12O6 ->[Zymase] 2C2H5OH + 2CO2}\]
In wine making, grapes are the source of sugars and yeast. As grapes ripen, the quantity of sugar increases and yeast grows on the outer skin. When grapes are crushed, sugar and the enzyme come in contact and fermentation starts. Fermentation takes place in anaerobic conditions i.e. in absence of air. Carbon dioxide is released during fermentation.
APPEARS IN
RELATED QUESTIONS
Write the main product(s) in each of the following reactions:
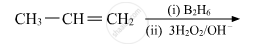
Name the reagents used in the following reactions:
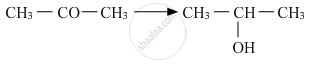
What is meant by hydroboration-oxidation reaction? Illustrate it with an example.
Name the reagent used in the following reaction:
Dehydration of propan-2-ol to propene.
Name the reagents used in the following reactions:

Primary alcohols are prepared by the reduction of carboxylic acids. Though lithium aluminium hydride is a strong reducing agent, it is not used in the reaction. This is so because:
Commercially carboxylic acids are reduced to alcohols by converting them to the ______.
The Wittig reaction is a reaction between a carbonyl compound (aldehyde or ketone only) and a species known as a phosphoniumylide. What is the expected final product in the Wittig reaction?
An aldehyde isomeric with allyl alcohol gives phenyl hydrazone. Pick out a ketone that too gives a phenyl hydrazone containing the same percentage of nitrogen.
How are the following conversion carried out?
\[\ce{Methyl magnesium bromide -> 2-Methylpropan-2-ol}\]
