Advertisements
Advertisements
Question
Name the reagent used in the following reaction:
Butan-2-one to butan-2-ol.
Solution
H2/Ni or NaBH4 or LiAIH4 is used as a reagent in butan-2-one to butan-2-ol.
APPEARS IN
RELATED QUESTIONS
How will you convert: Propene to Propan-2-ol?
Name the reagents used in the following reactions:

Name the reagents used in the following reactions:
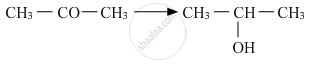
Write the structures of the main products when acetone (CH3 − CO − CH3) reacts with the following reagents :
CH3MgBr and then H3O+
Predict the major product of acid catalysed dehydration of 1-methylcyclohexanol.
How is the following conversion carried out?
\[\ce{Propene -> Propan-2-ol}\]
How will you convert: Phenol to 2, 4, 6 − trinitrophenol?
Name the reagents used in the following reactions:

Write the structures of A, B and C in the following reactions :
Monochlorination of toluene in sunlight followed by hydrolysis by aq. \[\ce{NaOH}\] yields.
Commercially carboxylic acids are reduced to alcohols by converting them to the ______.
Carboxylic acids are more acidic than phenol and alcohol because of
The reagent used for dehydration of an alcohol is
Most readily hydrolysed halide is:-
When glycol is heated with dicorboxylic acid the product are

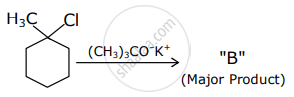
The products "A" and "B" formed in above reactions are:
An aldehyde isomeric with allyl alcohol gives phenyl hydrazone. Pick out a ketone that too gives a phenyl hydrazone containing the same percentage of nitrogen.
Given below are two statements:
Statement I: On heating with KHSO4, glycerol is dehydrated and acrolein is formed.
Statement II: Acrolein has a fruity odour and can be used to test glycerol's presence.
Choose the correct option.
How are the following conversion carried out?
\[\ce{Methyl magnesium bromide -> 2-Methylpropan-2-ol}\]
