Advertisements
Advertisements
Question
Solve the following problem:
Your laboratory partner was given the task of measuring the length of a box (approx 5 in) as accurately as possible, using a metre stick graduated in milimeters. He supplied you with the following measurements:
12.65 cm, 12.6 cm, 12.65 cm, 12.655 cm, 126.55 mm, 12 cm.
State which of the measurements you would accept, giving the reason.
Solution
The metre stick is graduated in millimetres i.e. 1 mm to 1000 mm, and 1 mm = 0.1 cm. Therefore, if length is measured in centimetres, the least count of metre stick is 0.1 cm. The results 12.6 cm has the least count of 0.1 cm and is acceptable result.
APPEARS IN
RELATED QUESTIONS
Explain the term Saturated solution giving examples.
Calculate the amount of carbon dioxide that could be produced when 1 mole of carbon is burnt in 16 g of dioxygen.
Calculate the amount of carbon dioxide that could be produced when 2 moles of carbon are burnt in 16 g of dioxygen.
What is the concentration of sugar (C12H22O11) in mol L–1 if its 20 g are dissolved in enough water to make a final volume up to 2 L?
Calculate the molarity of a solution of ethanol in water in which the mole fraction of ethanol is 0.040 (assume the density of water to be one).
To make a saturated solution, 36 g of sodium chloride is dissolved in 100 g of water at 293 K. Find its concentration at this temperature.
Explain the term mole fraction
Solve the following problem:
Write the following number in ordinary decimal form:
3.49 × 10−11
Solve the following problem:
Write the following number in ordinary decimal form:
5.16 × 104
Solve the following problem:
Write the following number in ordinary decimal form:
0.011 × 10−3
Solve the following problem:
Write the following number in ordinary decimal form:
5.00858585
Solve the following problem:
Perform the following calculation. Round off your answer to three digits.
(8.39 × 107) × (4.53 × 109)
Solve the following problem:
Perform the following calculation. Round off your answer to three digits.
`(8.94xx10^6)/(4.35xx10^4)`
Solve the following problem:
The hourly energy requirements of an astronaut can be satisfied by the energy released when 34 grams of sucrose are “burnt” in his body. How many grams of oxygen would be needed to be carried in space capsule to meet his requirement for one day?
Name the process associated with the following
A drop of ink placed on the surface of water contained in a glass spreads throughout the water.
Non-metals are usually poor conductors of heat and electricity. They are non-lustrous, non-sonorous, non-malleable and are coloured.
Name a lustrous non-metal.
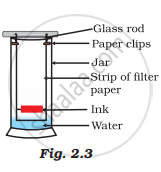
A child wanted to separate the mixture of dyes constituting a sample of ink. He marked a line by the ink on the filter paper and placed the filter paper in a glass containing water as shown in Fig.2.3. The filter paper was removed when the water moved near the top of the filter paper.
(i) What would you expect to see, if the ink contains three different coloured components?
(ii) Name the technique used by the child.
(iii) Suggest one more application of this technique.
Calculate the mass of sodium sulphate required to prepare its 20% (mass percent) solution in 100g of water?
If 500 mL of a 5 M solution is diluted to 1500 mL, what will be the molarity of the solution obtained?
What will be the molality of the solution containing 18.25 g of \[\ce{HCl}\] gas in 500 g of water?
Which of the following terms are unitless?
(i) Molality
(ii) Molarity
(iii) Mole fraction
(iv) Mass percent
What is the difference between molality and molarity?
The molarity of pure water is ______.
An aqueous KCl solution of density 1.20 g mL-1 has a molality of 3.30 mol kg-1. The molarity of the solution in mol L-1 is ______. (Nearest integer)
Find the molality of solution if boiling point increases by 1.75 K and molal elevation constant of solvent is 5K kg mol-1.
