Advertisements
Advertisements
Question
Some elements and their atomic radii are given here. Arrange them in decreasing order of their atomic radii. Identify which of the above elements is the biggest atom and which is smallest?
| Element | K | Na | Rb | Cs | Li |
| Atomic radius (pm) | 231 | 186 | 244 | 262 | 151 |
Solution
- Decreasing order of atomic radii: Cs > Rb > K > Na > Li
- Cesium (Cs) has the biggest atom and lithium (Li) has the smallest atom.
APPEARS IN
RELATED QUESTIONS
Answer the following in respect of element `31/15 P `
Is it a reducing agent or oxidizing agent?
Fill in the blank:
On moving across a period from right to left in the periodic table, the atomic size of the atom ___________.
Write scientific reason.
Atomic radius goes on increasing down a group.
Study the radius of the element given below and answer the following questions.
| elements | K | Na | Rb | Cs | Li |
| Atomic radius (pm) | 231 | 186 | 244 | 262 | 151 |
a) Which of the above elements have the smallest atom?
b) In which group of the modern periodic table the above element are belongs?
c) What is the periodic trend observed in the variation of atomic radii down a group?
What happens to the atomic size of elements on moving from left to right in a period?
The changes in the properties of elements on moving from left to right across a period of the Periodic Table. For the property, choose the correct answer.
The atomic size:
On moving from left to right in a periodic table, the size of the atom _______.
The size of an atom is indicated by its _______.
The size of an atom depends on the number of valence electrons.
Write an Explanation.
Atomic radius
Write information about the given atomic numbers in the table. 10, 20, 7.
| Atomic Number | Electronic configuration | Group | Period | Element |
| 10 | ||||
| 20 | ||||
| 7 |
Carbon belongs to the second period and Group 14. Silicon belongs to the third period and Group 14. If the atomic number of carbon is 6, the atomic number of silicon is ______
Which of the following is the correct order of atomic size?
Which of the following gives the correct increasing order of the atomic radii of O, F, and N?
Elements have been arranged in the following sequence on the basis of their increasing atomic masses.
| F, | Na, | Mg, | Al, | Si, | P, | S, | Cl, | Ar, | K |
- Pick two sets of elements which have similar properties.
- The given sequence represents which law of classification of elements?
- In below ladder symbols of elements are jumbled up. Rearrange these symbols of elements in the increasing order of their atomic number in the Periodic Table.
- Arrange them in the order of their group also.
- Electropositive nature of the element(s) increases down the group and decreases across the period
- Electronegativity of the element decreases down the group and increases across the period
- Atomic size increases down the group and decreases across a period (left to right)
- Metallic character increases down the group and decreases across a period.
On the basis of the above trends of the Periodic Table, answer the following about the elements with atomic numbers 3 to 9.
- Name the most electropositive element among them
- Name the most electronegative element
- Name the element with smallest atomic size
- Name the element which is a metalloid
- Name the element which shows maximum valency.
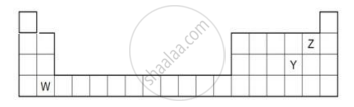
The diagram below shows part of the periodic table.
- Which elements would react together to form covalent compounds?
- Between the two elements W and Z, which will have a bigger atomic radius? Why?
Arrange the following as per the instruction given in the bracket:
Carbon, Fluorine, Beryllium (decreasing order of atomic size).
This question refers to the elements of the Periodic Table with atomic numbers from 3 to 18. Some of the elements are shown by letters, but the letters are not the usual symbols of the elements.
| 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| A | B | C | D | E | F | G | H |
| 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
| I | J | K | L | M | N | O | P |
Which of these have least atomic size in period 3?
