Advertisements
Advertisements
Question
Specific heat capacity of substance A is 3.8 J g-1K-1 whereas the specific heat capacity of substance B is 0.4 J g-1 K-1
(i) Which of the two is a good conductor of heat?
(ii) How is one led to the above conclusion?
(iii) If substances A and B are liquids then which one would be more useful in car radiators?
Solution
(i) Substance B is a good conductor out of the two substances.
(ii) The specific heat capacity of B is lower than A. This means that less heat is required to raise the temperature of 1 g of B by 1 K than the heat required for A.
(iii) If both substances were liquids, then substance A will be more useful in radiators. This is because A will extract more heat without much change in its temperature as it has high specific heat capacity.
APPEARS IN
RELATED QUESTIONS
Write the expression for the heat energy Q received by the substance when m kg of substance of specific heat capacity c Jkg-1 k-1 is heated through Δt° C.
Water is used in hot water bottles for fomentation. Give a reason.
State the impact of global warming on life on the earth.
104g of water at 30°C is taken in a calorimeter made of copper of mass 42 g. When a certain mass of ice at 0°C is added to it, the final steady temperature of the mixture after the ice has melted, was found to be 10°C. Find the mass of ice added. [Specific heat capacity of water = 4.2 Jg–1°C–1 ; Specific latent heat of fusion of ice = 336 Jg–1; Specific heat capacity of copper = 0.4 Jg–1°C–1] .
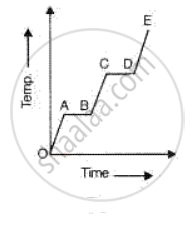
A substance is heated at a constant rate from a low temperature to a high temperature. A graph of temperature against time is shown in the figure. Which part or parts of the graph correspond(s) to the substance existing in two states?
Water boils at 120 °C in a pressure cooker. Explain the reason
How much heat energy is released when 5 g of water at 20° C changes to ice at 0° C?
[Specific heat capacity of water = 4.2 J g-1 ° C-1 Specific latent heat of fusion of ice = 336 J g-1]
Derive an expression for finding out the specific heat capacity of a body (solid) from the readings of an experiment given below:
(i) Mass of empty calorimeter (with stirrer) = m1 gm
(ii) Mass of the metal piece = M gm
(iii) Mass of colorimeter and water = m2 gm
(iv) Initial temperature and water = t1°C
(v) Temperature of hot solid (metal piece) = t2 °C
(vi) Final temperature of the mixture = t°C
(vii) Specific heat of calorimeter = 0.4 J gm / °C
Solve the following problem.
Specific latent heat of vaporization of water is 2.26 × 106 J/kg. Calculate the energy needed to change 5.0 g of water into steam at 100 ºC.
50 g of copper is heated to increase its temperature by 10° C. If the same quantity of heat is given to 5 g water, the rise in its temperature is [Specific heat of copper = 420 joule-kg-1 °C-1 , specific heat of water = 4200 joule-kg-I °C-1]
