Advertisements
Advertisements
Question
The reason for using Aluminium in the alloy duralumin is ______.
Options
Aluminium is brittle
Aluminium gives strength
Aluminium brings lightness
Aluminium lowers melting point
Solution
The reason for using Aluminium in the alloy duralumin is Aluminium brings lightness.
Explanation:
An alloy of aluminium, duralumin is used in making aircraft as it is light and corrosion-resistant.
APPEARS IN
RELATED QUESTIONS
Aluminium is a more active metal than iron, but suffers less corrosion. Why?
Name the constituents of Bronze.
Aluminium is a more active metal than iron, but suffers less corrosion. Why?
A to F below relate to the source and extraction of either zinc or aluminium.
- Bauxite
- Coke
- Cryolite
- Froth floatation
- Sodium hydroxide solution
- Zinc blende
Fill in the blanks using the most appropriate words from A to F:
(i) The ore from which aluminium is extracted must be treated with ______ so that pure aluminium oxide can be obtained.
(ii) Pure aluminium oxide is dissolved in ______ to make a conducting solution.
Describe the role played in the extraction of aluminum:
Cryolite
Explain why it is preferably to use a number of graphite electrodes as anode instead of a single electrode, during the above electrolysis.
Name the alloy used for the following purpose.
Aircraft
Name the compound formed when: Bauxite reacts with sodium hydroxide.
Write the balanced chemical equation to show the concentration of ore in Baeyer’s process.
Aluminium hydroxide to alumina
The following sketch illustrates the process of conversion of Alumina to Aluminium:
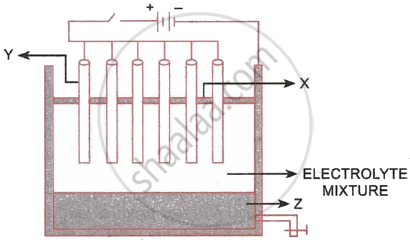
- Name the constituent of the electrolyte mixture which has a divalent metal in it.
- Name the powdered substances ‘X’ sprinkled on the surface of the electrolyte mixture.
- What is the name of the process?
- Write the reactions taking place at the electrodes ‘Y’ (anode) and ‘Z’ (cathode), respectively.
