Advertisements
Advertisements
Question
Use Bohr's postulate to prove that the radius of nth orbit in a hydrogen atom is proportional to n2.
Solution
The necessary centripetal force for the rotation of an electron is supplied by the electrostatic force between the electron and the nucleus.
`"mv"^2/"r" = (1/(4πε_0))("e"^2/"r"^2)` ....[putting Z = 1]
Or, mv2 = `"e"^2/(4πε_0"r")` .....(i)
From Bohr's theory,
mvr = `"nh"/(2π)`
∴ v = `"nh"/(2π"mr")`
Putting in equation (i)
`"m"("nh"/(2π"mr"))^2 = "e"^2/(4πε_0"r")`
Or, r = `(ε_0"n"^2"h"^2)/(π"me"^2)`
In general,
rn = `(ε_0"n"^2"h"^2)/(π"me"^2)`
∴ `"rn" ∝ "n"^2`
APPEARS IN
RELATED QUESTIONS
(i) State Bohr's quantization condition for defining stationary orbits. How does the de Broglie hypothesis explain the stationary orbits?
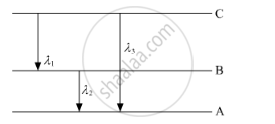
(ii) Find the relation between three wavelengths λ1, λ2 and λ3 from the energy-level diagram shown below.
If the photon of the wavelength 150 pm strikes an atom and one of its inner bound electrons is ejected out with a velocity of 1.5 × 107 ms–1, calculate the energy with which it is bound to the nucleus.
The difference in the frequencies of series limit of Lyman series and Balmer series is equal to the frequency of the first line of the Lyman series. Explain.
When a photon stimulates the emission of another photon, the two photons have
(a) same energy
(b) same direction
(c) same phase
(d) same wavelength
Suppose in an imaginary world the angular momentum is quantized to be even integral multiples of h/2π. What is the longest possible wavelength emitted by hydrogen atoms in visible range in such a world according to Bohr's model?
According to Bohr's theory, an electron can move only in those orbits for which its angular momentum is integral multiple of ____________.
For the ground state, the electron in the H-atom has an angular momentum = h, according to the simple Bohr model. Angular momentum is a vector and hence there will be infinitely many orbits with the vector pointing in all possible directions. In actuality, this is not true ______.
The inverse square law in electrostatics is |F| = `e^2/((4πε_0).r^2)` for the force between an electron and a proton. The `(1/r)` dependence of |F| can be understood in quantum theory as being due to the fact that the ‘particle’ of light (photon) is massless. If photons had a mass mp, force would be modified to |F| = `e^2/((4πε_0)r^2) [1/r^2 + λ/r]`, exp (– λr) where λ = mpc/h and h = `h/(2π)`. Estimate the change in the ground state energy of a H-atom if mp were 10-6 times the mass of an electron.
The ground state energy of hydrogen atoms is -13.6 eV. The photon emitted during the transition of electron from n = 3 to n = 1 unknown work function. The photoelectrons are emitted from the material with a maximum kinetic energy of 9 eV. Calculate the threshold wavelength of the material used.
Hydrogen atom from excited state comes to the ground state by emitting a photon of wavelength λ. If R is the Rydberg constant then the principal quantum number n of the excited state is ______.
