Advertisements
Advertisements
Question
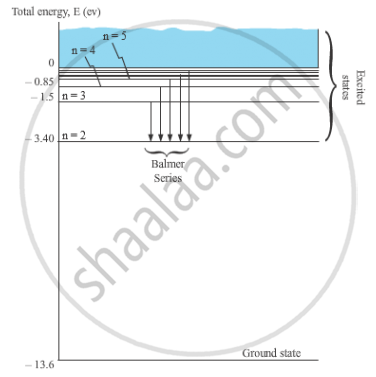
Using Bohr’s postulates, obtain the expression for the total energy of the electron in the stationary states of the hydrogen atom. Hence draw the energy level diagram showing how the line spectra corresponding to Balmer series occur due to transition between energy levels.
Solution
According to Bohr’s postulates, in a hydrogen atom, a single alectron revolves around a nucleus of charge +e. For an electron moving with a uniform speed in a circular orbit os a given radius, the centripetal force is provided by Columb force of attraction between the electron and the nucleus. The gravitational attraction may be neglected as the mass of electron and proton is very small.
So,
`(mv^2)/r = (ke^2)/r^2`
or `mv^2 = (ke^2)/r .............. (1)`
where m = mass of electron
r = radius of electronic orbit
v = velocity of electron.
Again,
`mvr = (nh)/(2π)`
or `v = (nh)/(2πmr)`
From eq.(1), we get,
`m((nh)/(2πmr)^2) = (ke^2)/r`
`=> r = (n^2h^2)/(4π^2kme^2).....................(2)`
(i) Kinetic energy of electron,
`E_k = 1/2 mv^2 = (ke^2)/(2r)`
Using eq (2), we get
`Ek =ke^2/2 (4π^2kme^2)/(n^2h^2)`
=`(4π^2kme^2)/(n^2h^2)`
`(2π^2k^2me^4)/(n^2h^2)`
(ii) Potential energy
`E_p = -(k(e) xx (e))/r = - (ke^2) / r `
Using eq (2), we get
`E^p =-ke^2 xx (4π^2kme^2)/(n^2h^2)`
= `-(4π^2k^2me^4)/(n^2h^2)`
Hence, total energy of the electron in the nth orbit
`E =E_p+E_k =(4π^2k^2me^4)/(n^2h^2)+(2π^2k^2me^4)/(n^2h^2) =- (2π^2k^2me^4)/(n^2h^2) =- (13.6)/n^2 eV `
When the electron in a hydrogen atom jumps from higher energy level to the lower energy level, the difference of energies of the two energy levels is emitted as a radiation of particular wavelength. It is called a spectral line.
In H-atom, when an electron jumps from the orbit ni to orbit nf, the wavelength of the emitted radiation is given by,
`1/λ = R (1/n_f^2 -1/n_i^2)`
Where,
R → Rydberg’s constant = 1.09678 ×107 m−1
For Balmer series, nf = 2 and ni = 3, 4, 5, …
`1/λ = R (1/2^2 -1/n_i^2)`
Where, ni = 3, 4, 5, …
These spectral lines lie in the visible region.
APPEARS IN
RELATED QUESTIONS
The radius of the innermost electron orbit of a hydrogen atom is 5.3 × 10−11 m. What are the radii of the n = 2 and n = 3 orbits?
When a photon is emitted by a hydrogen atom, the photon carries a momentum with it. (a) Calculate the momentum carries by the photon when a hydrogen atom emits light of wavelength 656.3 nm. (b) With what speed does the atom recoil during this transition? Take the mass of the hydrogen atom = 1.67 × 10−27 kg. (c) Find the kinetic energy of recoil of the atom.
In Bohr model of hydrogen atom, which of the following is quantised?
Derive an expression for the frequency of radiation emitted when a hydrogen atom de-excites from level n to level (n – 1). Also show that for large values of n, this frequency equals to classical frequency of revolution of an electron.
The mass of a H-atom is less than the sum of the masses of a proton and electron. Why is this?
The inverse square law in electrostatics is |F| = `e^2/((4πε_0).r^2)` for the force between an electron and a proton. The `(1/r)` dependence of |F| can be understood in quantum theory as being due to the fact that the ‘particle’ of light (photon) is massless. If photons had a mass mp, force would be modified to |F| = `e^2/((4πε_0)r^2) [1/r^2 + λ/r]`, exp (– λr) where λ = mpc/h and h = `h/(2π)`. Estimate the change in the ground state energy of a H-atom if mp were 10-6 times the mass of an electron.
Given below are two statements:
Statements I: According to Bohr's model of an atom, qualitatively the magnitude of velocity of electron increases with decrease in positive charges on the nucleus as there is no strong hold on the electron by the nucleus.
Statement II: According to Bohr's model of an atom, qualitatively the magnitude of velocity of electron increase with a decrease in principal quantum number.
In light of the above statements, choose the most appropriate answer from the options given below:
Find the ratio of energies of photons produced due to transition of an election of hydrogen atom from its (i) second permitted energy level to the first level. and (ii) the highest permitted energy level to the first permitted level.
An electron in a hydrogen atom has an energy of -3.4 eV. The difference between its kinetic and potential energy is ______.
The energy of an electron in the nth orbit of the hydrogen atom is En = -13.6/n2eV. The negative sign of energy indicates that ______.
