Advertisements
Advertisements
Question
Using Bohr’s postulates, obtain the expressions for (i) kinetic energy and (ii) potential energy of the electron in stationary state of hydrogen atom.
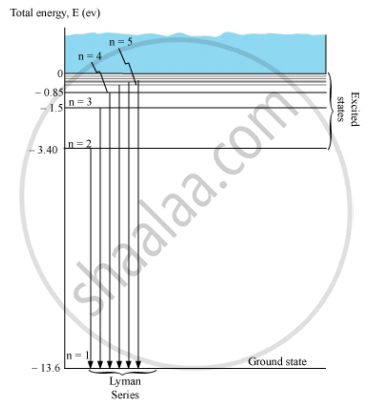
Draw the energy level diagram showing how the transitions between energy levels result in the appearance of Lymann Series.
Solution
According to Bohr’s postulates, in a hydrogen atom, a single alectron revolves around a nucleus of charge +e. For an electron moving with a uniform speed in a circular orbit os a given radius, the centripetal force is provided by Columb force of attraction between the electron and the nucleus. The gravitational attraction may be neglected as the mass of electron and proton is very small.
So,
`mv^2/r = (ke^2)/r^2`
0r `mv^2 = (ke^2)/r ...................(1)`
where m = mass of electron
r = radius of electronic orbit
v = velocity of electron.
Again,
`mvr = (nh)/(2π)`
` or v = (nh)/(2πmr)`
From eq(1), we get,
`m ((nh)/(2πmr))^2 = (ke^2)/r`
`⇒ r = (n^2h^2)/(4π^2kme^2) .....................(2) `
(i) Kinetic energy of electron,
`E_k = 1/2mv^2 = (ke^2)/(2r)`
Using eq (2), we get
`E_k = (ke^2)/2 (4π^2kme^2)/(n^2h^2)`
= `(2π^2k^2me^4)/(n^2h^2)`
(ii) Potential energy
`E^p= - ke^2 xx (4π^2k^2me^4)/(n^2h^2) `
Energy level diagram showing the transitions between energy levels result in the appearance ofLymann series:
For Lymann series, nf = 1 and ni = 2, 3, 4, 5, …
`1/λ = R_H(1/I^2 - 1/n^2)`
Where, ni = 2, 3, 4, 5, …
APPEARS IN
RELATED QUESTIONS
According to Bohr, 'Angular momentum of an orbiting electron is quantized'. What is meant by this statement?
If l3 and l2 represent angular momenta of an orbiting electron in III and II Bohr orbits respectively, then l3: l2 is :
Draw energy level diagram for a hydrogen atom, showing the first four energy levels corresponding to n=1, 2, 3 and 4. Show transitions responsible for:
(i) Absorption spectrum of Lyman series.
(ii) The emission spectrum of the Balmer series.
Calculate the energy and frequency of the radiation emitted when an electron jumps from n = 3 to n = 2 in a hydrogen atom.
A set of atoms in an excited state decays ______.
The inverse square law in electrostatics is |F| = `e^2/((4πε_0).r^2)` for the force between an electron and a proton. The `(1/r)` dependence of |F| can be understood in quantum theory as being due to the fact that the ‘particle’ of light (photon) is massless. If photons had a mass mp, force would be modified to |F| = `e^2/((4πε_0)r^2) [1/r^2 + λ/r]`, exp (– λr) where λ = mpc/h and h = `h/(2π)`. Estimate the change in the ground state energy of a H-atom if mp were 10-6 times the mass of an electron.
An electron in H-atom makes a transition from n = 3 to n = 1. The recoil momentum of the H-atom will be ______.
According to Bohr atom model, in which of the following transitions will the frequency be maximum?
What is meant by ionisation energy?
State the Bohr's postulate of angular momentum of an electron.
