Advertisements
Advertisements
Question
What happens when an acid reacts with a metal?
Solution
When a metal is treated with an acid, it liberates hydrogen gas from the acid and combines with the remaining part of the acid to form a compound called a salt.
APPEARS IN
RELATED QUESTIONS
How is the concentration of hydronium ions (H3O+) affected when a solution of an acid is diluted?
On adding dilute hydrochloric acid to copper oxide powder, the solution formed is blue-green.
On the basis of the above reaction, what can you say about the nature of copper oxide?
Name the gas evolved when zinc granules are treated/heated with:
hydrochloric acid solution
What would be the colour of litmus in a solution of sodium carbonate?
Complete and balance the following chemical equations:
`Ca(OH)_2 + Cl_2 ->`
Explain why, sodium hydrogencarbonate is used as an antacid.
In an attempt to demonstrate electrical conductivity through an electrolyte, the apparatus setup. Which among the following statement(s) is(are) correct?
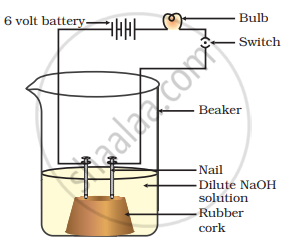
- Bulb will not glow because electrolyte is not acidic
- Bulb will glow because NaOH is a strong base and furnishes ions for conduction.
- Bulb will not glow because circuit is incomplete
- Bulb will not glow because it depends upon the type of electrolytic solution
Which acid is present in milk?
Are all acids corrosive in nature? Name a few acids which are non-corrosive and may be part of our food.
Consider the following salt:
NH4X
If in salt NH4X, X is nitrate, then its solution will give what colour with a universal indicator? Why?
