Advertisements
Advertisements
Question
What is Hinsberg's reagent?
Solution 1
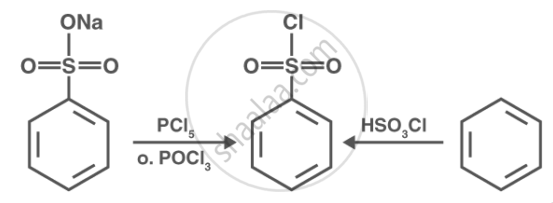
Hinsberg reagent is also known as benzene sulphonyl chloride \[\ce{(C6H5SO2Cl)}\]. When it reacts with primary and secondary amines, it produces sulphonamides. Allowing secondary and tertiary amines to react with Hinsberg's reagent allows them to be distinguished (benzesulphonyl chloride \[\ce{C6H5SO2Cl}\]). Secondary amines react with Hinsberg's reagent to form an alkali-insoluble product. \[\ce{N}\], \[\ce{N}\]-diethylamine for example, reacts with Hinsberg's reagent to form \[\ce{N}\], \[\ce{N}\]-diethlbenzenesulphonamide, which is insoluble in alkalis. Tertiary amines, on the other hand, are unaffected by Hinsberg's reagent.
Solution 2
The Hinsberg reagent is a chemical reagent used in organic chemistry for the identification and differentiation of primary, secondary, and tertiary amines. The Hinsberg reagent is typically composed of benzenesulfonyl chloride \[\ce{(C6H5SO2Cl)}\].
Notes
Students can refer to the provided solutions based on their preferred marks.
APPEARS IN
RELATED QUESTIONS
Give a simple chemical test to distinguish between the following pair of compounds :
(CH3)2NH and (CH3)3N
Account for the following:
On reaction with benzene sulphonyl chloride, primary amine yields product soluble in alkali whereas secondary amine yields product insoluble in alkali.
Write chemical equation in support of your answer.
Out of  Cl and O2N
Cl and O2N  Cl. which one is more reactive towards nucleophilic substitution reaction and why?
Cl. which one is more reactive towards nucleophilic substitution reaction and why?
Hinsberg’s reagent reacts with primary and secondary amines to form sulphonamides. This reagent is also known as:
p-toluenesulphonyl chloride does not react with ____________.
p-toluenesulphonyl chloride is used to:
The Hiitsberg's method is used for which of the following?
Give reasons for the following observation:
Primary amine on treatment with benzenesulphonyl chloride forms a product which is soluble in NaOH however secondary amine gives product which is insoluble in NaOH.
A primary amine 'A' C2H7N reacts with alkyl halide (C2H5l) to give secondary amine 'B'. 'B' reacts with C6H5SO2Cl to give a solid 'C' which is insoluble in alkali. Identify 'A', 'B', 'C' and write all the chemical reaction involved.
