Advertisements
Advertisements
Question
What is Nessler’s reagent ?
Solution
- Nessler's reagent is a solution of mercury(II) iodide (HgI2) in potassium iodide (KI) and potassium hydroxide (KOH) named after the German chemist Julius Nessler
- Nessler’s reagent is an alkaline solution of K2 HgI4
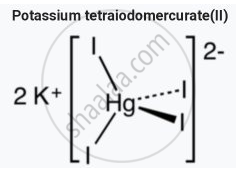
- It is used in testing for ammonia, with which it forms a brown coloration or precipitate.
APPEARS IN
RELATED QUESTIONS
Describe the laboratory method of preparation of ammonia
What is the action of Excess of air on ammonia ?
What is the action of the following reagents on ammonia :
Nessler's reagent
Mention the conditions required to maximise the yield of ammonia.
How does ammonia react with a solution of Cu2+?
What happens when (NH4)2Cr2O7 is heated? Write the equations.
What is the action of Excess of chlorine on ammonia?
What is the action of Na Metal on ammonia?
What is the action of the following reagents on ammonia :
Sodium metal
Ammonia has a higher boiling point and is less volatile because of ____________.
Which compound is used in the preparation of caprolactam?
Ammonia act as a Lewis base because nitrogen has ____________.
In Haber’s process for the manufacture of NH3:
Ammonia on reaction with hypochlorite anion can form:
The shape of ammonia molecule is ____________.
Liquid ammonia bottles are opened after cooling them in ice for some time. It is because liquid NH3 ____________.
Ammonia is generally manufactured for fertilizers by the reaction:
Which one of the following is not a use of ammonia?
A brown ring is formed in the ring test for \[\ce{NO3^{-}}\] ion. It is due to the formation of ______.
In the preparation of HNO3, we get NO gas by catalytic oxidation of ammonia. The moles of NO produced by the oxidation of two moles of NH3 will be ______.
\[\ce{PCl5}\] reacts with finely divided silver on heating and a white silver salt is obtained, which dissolves on adding excess aqueous \[\ce{NH3}\] solution. Write the reactions involved to explain what happens.
What happens when reactions:
Benzylchloride is treated with ammonia followed by the reaction with Chloromethane.
