Advertisements
Advertisements
Question
When BCl3 is treated with water, it hydrolyses and forms [B[OH]4]– only whereas AlCl3 in acidified aqueous solution forms [Al(H2O)6]3+ ion. Explain what is the hybridisation of boron and aluminium in these species?
Solution
\[\ce{BCl3 + 3H2O -> B(OH)3 + 3HCl}\]
\[\ce{B(OH)3 + H2O -> [B(OH)4]– + H+}\]
B(OH)3 due to its incomplete octet accepts an electron pair (as OH–) to give [B(OH)4]–. Boron in this ion involves one 2s orbital and three 2p orbitals. Thus, hybridisation of B in [B(OH)4]– is sp3.
\[\ce{AlCl3 + 6H2O ->[HCl] [Al(H2O)6]^{3+} + 3Cl-}\]
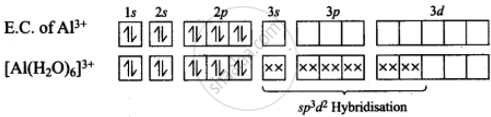
Hence, hybridisation of Al is sp3d2.
APPEARS IN
RELATED QUESTIONS
If B–Cl bond has a dipole moment, explain why BCl3 molecule has zero dipole moment.
Aluminium trifluoride is insoluble in anhydrous HF but dissolves on the addition of NaF. Aluminium trifluoride precipitates out of the resulting solution when gaseous BF3 is bubbled through. Give reasons.
What happens when BF3 is reacted with ammonia?
What do you understand by inert pair effect?
Write a balanced equation for B2H6 + NH3 → ?
Explain the following:
PbX2 is more stable than PbX4.
Identify the compounds A, X and Z in the following reactions:
\[\ce{X ->[Δ][370 K] HBO2 ->[Δ][> 370 K] Z}\]
Describe the general trends in the following properties of the elements in Groups 13 and 14.
Ionisation enthalpy
Account for the following observations:
PbO2 is a stronger oxidising agent than SnO2
BCl3 exists as monomer whereas AlCl3 is dimerised through halogen bridging. Give reason. Explain the structure of the dimer of AlCl3 also.
