Advertisements
Advertisements
Question
When ethanol reacts with ethanoic acid in the presence of conc. H2SO4, a substance with fruity smell is produced. Answer the following:-
(i) State the class of compounds to which the fruity smelling compounds belong. Write the chemical equation for the reaction and write the chemical name of the product formed.
(ii) State the role of conc. H2SO4 in the reaction.
Solution
(i). An ester is formed when an alcohol reacts with a carboxylic acid in the presence of an acidic medium. An ester has a fruity smell.
The chemical equation for the reaction between ethanol and ethanoic acid in the presence of conc. H2SO4 can be written as follows:-
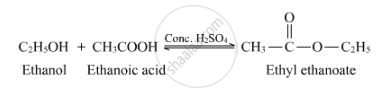
(ii). Concentrated sulphuric acid acts as a protonating catalyst during the esterification reaction.
RELATED QUESTIONS
While studying saponification reaction for the preparation of soap, a teacher suggested to a student to add a small quantity of common salt to the reaction mixture. The function of common salt in this reaction is to
(A) reduce the alkalinity of the soap
(B) reduce the acidity of the soap
(C) enhance the cleansing capacity of soap
(D) favour precipitation of soap
Draw the structure for the following compound.
Ethanoic acid
Name the gas evolved when ethanoic acid is added to sodium carbonate. How would you prove the presence of this gas?
Choose those compounds from the following which can turn blue litmus solution red:
HCHO, CH3COOH, CH3OH, C2H5OH, HCOOH, CH3CHO
Give reasons for your choice.
State any two uses of esters.
Choose the correct word/phrase from the options given below to complete the following sentence:
When acetaldehyde is oxidized with acidified potassium dichromate, it forms ______.
Ethanoic acid is also known as which of these?
When ethanoic acid is treated with NaHCO^ the gas evolved is ______.
Shristi heated Ethanol with a compound A in presence of a few drops of concentrated sulphuric acid and observed a sweet smelling compound B is formed. When B is treated with sodium hydroxide it gives back Ethanol and a compound C.
- Identify A and C
- Give one use each of compounds A and B.
- Write the chemical reactions involved and name the reactions.
Give the balanced chemical equation of the following reaction:
Neutralization of NaOH with ethanoic acid.
