Advertisements
Advertisements
Question
When fused lead bromide is electrolyzed we observe
Options
A silver grey deposit at anode and a reddish brown deposit at cathode
A silver grey deposit at cathode and reddish brown deposit at anode
A silver grey deposit at cathode and reddish brown fumes at anode
Silver grey fumes at anode and reddish brown fumes at cathode
Solution
A silver grey deposit at the cathode and reddish brown fumes at the anode.
APPEARS IN
RELATED QUESTIONS
State the observations at the anode and at the cathode during the electrolysis of fused lead bromide using graphite electrodes
Define the following term:
Non-electrolyte
Explain the term : Anode mud
What are the particles present in a compound which is a non- electrolyte?
If a fused metallic chloride is electrolyzed, at which electrode would the metal be obtained?
Copper sulphate solution is electrolyzed using copper electrodes. Study the diagram given alongside and answer the questions that follow.
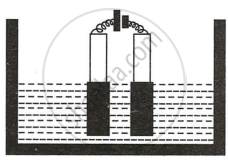
- Which electrode to your left or right is known as the oxidizing electrode and why?
- Write the equation representing the reaction that occurs.
- State two appropriate observations for the above electrolysis reactions.
Give appropriate scientific reasons for the following statement :
Electrolysis of molten lead bromide is considered to be a redox reaction.
Give reasons why sodium chloride will conduct electricity only in the fused or aqueous solution state.
An electrolyte which completely dissociates into ions is ______.
Classify the following substance:
Ammonium hydroxide
