Advertisements
Advertisements
Question
Which of the following are benzylic alcohols?
(i) \[\ce{C6H5 - CH2 - CH2OH}\]
(ii) \[\ce{C6H5 - CH2OH}\]
(iii) \[\begin{array}{cc}
\ce{C6H5 - CH - OH}\\
\phantom{}|\phantom{.}\\
\phantom{..}\ce{CH3}\phantom{}
\end{array}\]
(iv) \[\begin{array}{cc}
\ce{C6H5 - CH2 - CH - OH}\\
\phantom{.......}|\phantom{}\\
\phantom{.........}\ce{CH3}\phantom{}
\end{array}\]
Solution
(ii) \[\ce{C6H5 - CH2OH}\]
(iii) \[\begin{array}{cc}
\ce{C6H5 - CH - OH}\\
\phantom{}|\phantom{.}\\
\phantom{..}\ce{CH3}\phantom{}
\end{array}\]
Explanation:
In these alcohols, the –OH group is attached to a sp3- hybridised carbon atom next to an aromatic ring. For example,
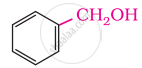
Primary
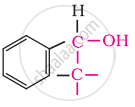
Secondary
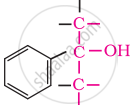
Tertiary
Allylic and benezlic alcohols may be primary, secondary or tertiary.
APPEARS IN
RELATED QUESTIONS
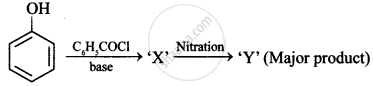
Write the main product(s) in each of the following reactions:
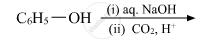
Picric acid is ____________.
On distilling phenol with Zn dust, one gets:
In the reaction of phenol with CHCl3 and aqueous NaOH at 343 K, the electrophile attacking the ring is:

Which of the following are used to convert \[\ce{RCHO}\] into \[\ce{RCH2OH}\]?
(i) \[\ce{H2/Pd}\]
(ii) \[\ce{LiAlH4}\]
(iii) \[\ce{NaBH4}\]
(iv) Reaction with \[\ce{RMgX}\] followed by hydrolysis
Convert the following:
Phenol to N-phenylethanamide.
Why ortho-nitrophenol is steam volatile while para-nitrophenol is not?
Write the name of the reaction, structure and IUPAC name of the product formed when:
Phenol reacts with CHCl3 in the presence of NaOH followed by hydrolysis.
