Advertisements
Advertisements
Question
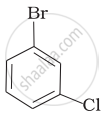
Why is the boiling point of o-dichlorobenzene higher than p-dichlorobenzene, but the melting point of para-isomer is higher than ortho-isomer?
Solution
Because o-dichlorobenzene has a greater dipole moment and a greater dipole-dipole interaction than p-dichlorobenzene, it has a higher boiling point.
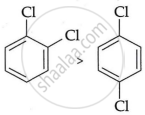
Boiling point
As opposed to ortho- and meta-isomer, p-dichlorobenzene has a higher melting point than that of o- and m-isomer due to its greater symmetry, which suits the crystal better.
APPEARS IN
RELATED QUESTIONS
Arrange the set of compounds in order of increasing boiling points.
1-Chloropropane, Isopropyl chloride, 1-Chlorobutane.
Define racemic mixture.
Arrange the following compounds in the increasing order of their densities.
(a)
(b)
(c)
(d)
Arrange the following compounds in increasing order of their boiling points.
(a) \[\begin{array}{cc}
\ce{CH3}\phantom{.................}\\
\backslash\phantom{.............}\\
\ce{CH - CH2Br}\\
/\phantom{.............}\\
\ce{CH3}\phantom{.................}
\end{array}\]
(b) \[\ce{CH3CH2CH2CH2Br}\]
(c) \[\begin{array}{cc}
\phantom{...}\ce{CH3}\\
\phantom{}|\\
\ce{H3C - C - CH3}\\
\phantom{}|\\
\phantom{..}\ce{Br}
\end{array}\]
Reaction of \[\ce{C6H5CH2Br}\] with aqueous sodium hydroxide follows ______.
Assertion: The boiling points of alkyl halides decrease in the order:
\[\ce{RI > RBr > RCl > RF}\]
Reason: The boiling points of alkyl chlorides, bromides and iodides are considerably higher than that of the hydrocarbon of comparable molecular mass.
Which out of the following is an intensive property?
Write the structure of the following organic halogen compound.
1,4-Dibromobut-2-ene
Name the following halides according to the IUPAC system and classify them as alkyl, allyl, benzyl (primary, secondary, tertiary), vinyl or aryl halide:
\[\ce{CH3 C(C2H5)2CH2Br}\]
Write the structure of the following organic halogen compound.
4-tert-Butyl-3-iodoheptane
