Advertisements
Advertisements
Question
Define racemic mixture.
Solution
Recemic mixture:
When equimolar quantities of dexto and leavo isomer are mixed ,then resulting mixture is found to be optically inactive due to external compensation .such optically inactive mixture known as recemate or recemic mixture.
APPEARS IN
RELATED QUESTIONS
Arrange the set of compounds in order of increasing boiling points.
Bromomethane, Bromoform, Chloromethane, Dibromomethane.
Arrange the set of compounds in order of increasing boiling points.
1-Chloropropane, Isopropyl chloride, 1-Chlorobutane.
Explain why alkyl halides, though polar, are immiscible with water?
p-Dichlorobenzene has higher m.p. and lower solubility than those of o- and m-isomers. Discuss.
Why dextro and laevorotatory isomers of Butan-2-ol are difficult to separate by fractional distillation?
For the same alkyl group, an alkyl bromide has a higher boiling point than alkyl fluoride because:
How many structural isomers are possible for a compound with the molecular formula C3H7Cl?
Which of the following is liquid at room temperature (b.p. is shown against it)?
Which of the following possesses the highest melting point?
Mg reacts with RBr best in ____________.
p-dichlorobenzene has a higher melting point than its o- and m-isomers because ____________.
Arrange the following compounds in the increasing order of their densities.
(a)
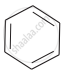
(b)
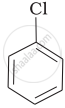
(c)
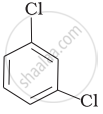
(d)
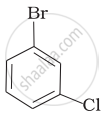
Arrange the following compounds in increasing order of their boiling points.
(a) \[\begin{array}{cc}
\ce{CH3}\phantom{.................}\\
\backslash\phantom{.............}\\
\ce{CH - CH2Br}\\
/\phantom{.............}\\
\ce{CH3}\phantom{.................}
\end{array}\]
(b) \[\ce{CH3CH2CH2CH2Br}\]
(c) \[\begin{array}{cc}
\phantom{...}\ce{CH3}\\
\phantom{}|\\
\ce{H3C - C - CH3}\\
\phantom{}|\\
\phantom{..}\ce{Br}
\end{array}\]
Out of o-and p-dibromobenzene which one has higher melting point and why?
Which of the following compounds will have the highest melting point and why?
| (I) |  |
|
(II) |
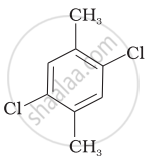 |
| (III) | 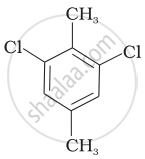 |
Assertion: The boiling points of alkyl halides decrease in the order:
\[\ce{RI > RBr > RCl > RF}\]
Reason: The boiling points of alkyl chlorides, bromides and iodides are considerably higher than that of the hydrocarbon of comparable molecular mass.
Which out of the following is an intensive property?
Why alkyl halides though polar are immiscible with water?
Arrange the isomeric dichlorobenzene in the increasing order of their boiling point and melting points.
Write the structure of the following organic halogen compound.
4-tert-Butyl-3-iodoheptane
Write the structure of the following organic halogen compound.
4-tert-Butyl-3-iodoheptane
Write the structure of the following organic halogen compound:
4-tert-Butyl-3-iodoheptane
Name the following halides according to the IUPAC system and classify them as alkyl, allyl, benzyl (primary, secondary, tertiary), vinyl or aryl halide:
\[\ce{CH3 C(C2H5)2CH2Br}\]
