Advertisements
Advertisements
Question
Write a word equation and then a balanced equation for the reaction taking place when:
Dilute hydrochloric acid reacts with magnesium ribbon.
Solution
- \[\ce{Magnesium ribbon + Hydrochloric acid ( dil{.}) -> Magnesium chloride + Hydrogen}\]
- \[\ce{Mg_{(s)} + 2HCl(dil{.}) -> MgCl2_{(aq)} + H2_{(g)}}\]
APPEARS IN
RELATED QUESTIONS
Why does an aqueous solution of an acid conduct electricity?
Dry HCl gas does not change the colour of dry blue litmus paper. Why?
Write a word equation and then a balanced equation for the reaction taking place when:
Dilute sulphuric acid reacts with aluminium powder.
Complete and balance the following chemical equations
Na2 CO3 (s) + HCI (aq) →
What ions are present in the solutions of following substances? (write the symbols only)
Magnesium hydroxide
A first-aid manual suggests that vinegar should be used to treat wasp stings and baking soda for bee stings. What does this information tell you about the chemical nature of:
bee stings?
What happens when a solution of sodium hydrogencarbonate is heated? Write equation of the reaction involved.
Choose the correct alternative and rewrite the following sentence.
Phenolphthalein is ___________ type of indicator.
Lime water reacts with chlorine to form:
A student added 10 g of calcium carbonate in a rigid container, secured it tightly and started to heat it. After some time, an increase in pressure was observed, the pressure reading was then noted at intervals of 5 mins and plotted against time, in a graph as shown below. During which time interval did maximum decomposition take place?
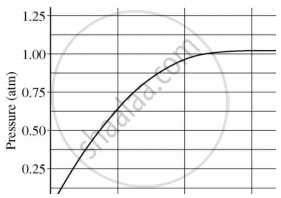
If a few drops of a concentrated acid accidentally spills over the hand of a student, what should be done?
In an attempt to demonstrate electrical conductivity through an electrolyte, the apparatus setup. Which among the following statement(s) is(are) correct?
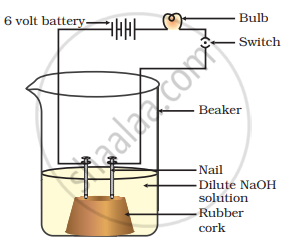
- Bulb will not glow because electrolyte is not acidic
- Bulb will glow because NaOH is a strong base and furnishes ions for conduction.
- Bulb will not glow because circuit is incomplete
- Bulb will not glow because it depends upon the type of electrolytic solution
Which of the following are present in a dilute aqueous solution of hydrochloric acid?
What happens when nitric acid is added to egg shell?
A salt may be ______.
Can we taste acids and bases to identify them?
Write notes on the properties of acids.
On placing a copper coin in a test tube containing green ferrous sulphate solution, it will be observed that the ferrous sulphate solution ______.
Consider the following salt:
\[\ce{ZCO3}\]
What would be the change in colour in blue litmus if \[\ce{ZCO3}\] is added to it and Z is potassium?
The metal that will not produce hydrogen gas when reacted with dilute acids.
