Advertisements
Advertisements
Question
Write the equation taking place at the anode.
Solution
Ag - e- → Ag+
Cu - e- → Cu2+
Cl⁻ ‒ e- → Cl
Cl + Cl → Cl2
APPEARS IN
RELATED QUESTIONS
State the observations at the anode and at the cathode during the electrolysis of fused lead bromide using graphite electrodes
Name the following:
An alloy of lead and tin that is used in electrical circuits.
Electrolysis of aqueous sodium chloride solution will form ________ at the cathode. (Hydrogen gas / Sodium metal)
Define the following term:
Non-electrolyte
A solution contains magnesium ions (Mg+2), iron (II) ions (Fe+2) and copper ions (Cu+2). On passing an electric current through this solution, which ions will be the first to be discharged at the cathode? Write the equation for the cathode reaction.
Fill in the blank from the choices given below :
Electro covalent compounds have a _____ boiling point
Copper sulphate solution is electrolyzed using copper electrodes. Study the diagram given alongside and answer the questions that follow.
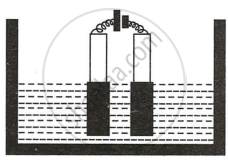
- Which electrode to your left or right is known as the oxidizing electrode and why?
- Write the equation representing the reaction that occurs.
- State two appropriate observations for the above electrolysis reactions.
Give appropriate scientific reasons for the following statement :
Carbon tetrachloride does not conduct electricity.
Give appropriate scientific reasons for the following statement :
The electrical conductivity of acetic acid is less in comparision to the electrical conductivity of dilute sulphuric acid at a given concentration.
Give reasons why sodium chloride will conduct electricity only in the fused or aqueous solution state.
