Advertisements
Advertisements
Question
Write reaction for preparation of acetophenone from benzoyl chloride.
Solution
\[\ce{\underset{\text{Benzoyl chloride}}{2C6H5 - COCl} + \underset{\text{Dimethyl cadmium}}{(CH3)2Cd} -> \underset{\text{Acetophenone}}{C6H5 - CO - CH3} + CdCl2}\]
RELATED QUESTIONS
Write the structure of the product formed when carboxylic acid is heated with a dehydrating agent like P2O5
Write the preparation reactions for acid amide from the following.
Carboxylic acid
Write reactions for the following conversions.
Propanone to Propane
Write chemical reactions to convert –COOH group of acetic acid into the following.
CH3COCl
Explain the acidic nature of carboxylic acids.
Write reactions for the action of the following reagents on p-chlorobenzaldehyde.
Ethane-1,2-diol in presence of dry HCl.
Complete the following sequence of reactions and write structures for A, B, C.
\[\ce{Dry ice ->[i. Dry ether][ii. Hydrolysis] A ->[PCl5] B ->[H2(gas)][Pd - BaSO4] C}\]
In the reaction,
\[\ce{CH3COOH ->[SOCl2] X ->[Sodium salt of carboxylic acid] Y}\].
The compound Y was found to be a mixed acid anhydride. Thus, the sodium salt of carboxylic acid used CANNOT be ____________.
In the following reaction:
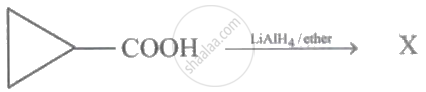
The product X is:
A flavouring agent found in oil of wintergreen is ______.
Products in the following reaction are:
\[\ce{CH3COOH + PCl3 ->[\Delta]}\]
Which of the following carboxylic acids will have the highest acidity?
Which among the following is the strongest acid?
Which of the following functional groups is reduced by diborane?
Which of the following compounds is obtained when ethanoic anhydride is treated with water?
Which of the following carboxylic acids ha highest acidic strength?
Which oxide of nitrogen is obtained on heating ammonium nitrate at 250°C?
The number of bonding pairs and lone pairs of electrons present in the central atom of ammonia molecular are respectively.
How sodium bicarbonate test is used to distinguish between carboxylic acid and phenol?
