Advertisements
Advertisements
Question
Explain the acidic nature of carboxylic acids.
Solution
Acidic nature of carboxylic acids:
- The carboxyl group (–COOH) imparts an acidic character to carboxylic acids.
- A carboxyl group is made of –OH group bonded to a carbonyl group.
- In aqueous solution, the H atom in OH of carboxyl group dissociates as proton and carboxylate ion is formed as the conjugate base,
\[\begin{array}{cc}
\phantom{.}\ce{O}\phantom{......................}\ce{O}\phantom{...........}\\
\phantom{.}||\phantom{......................}||\phantom{...........}\\
\ce{\underset{\text{Carboxylic acid}}{R - C -OH} + H2O ⇌ \underset{\text{Carboxylate ion}}{R - C - O-} + H3O+}
\end{array}\] - Carboxylate ion is resonance stabilized by two equivalent resonance structures as shown below.
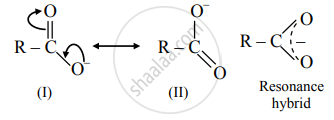
- Carboxylate ion has two resonance structures (I) and (II) and both of them are equivalent to each other.
- This gives good resonance stabilization to carboxylate ion, which in turn gives an acidic character to carboxylic acids.
APPEARS IN
RELATED QUESTIONS
Answer in brief.
Formic acid is stronger than acetic acid. Explain.
Arrange the following carboxylic acids with increasing order of their acidic strength and justify your answer.
1) ![]()
2) ![]()
3) ![]()
Write the reducing agent which CANNOT reduce –COOH group.
What is the action of following on proponal?
Hydroxyl amine
Write reactions for the following conversions.
Propanone to Propane
Write reactions for the following conversions.
4-Nitrobenzoic acid to Nitrobenzene
Write chemical reactions to convert –COOH group of acetic acid into the following.
CH4
Write chemical reactions to convert –COOH group of acetic acid into the following.
CH3COCl
Write reaction for preparation of acetophenone from benzoyl chloride.
In the reaction,
\[\ce{CH3COOH ->[SOCl2] X ->[Sodium salt of carboxylic acid] Y}\].
The compound Y was found to be a mixed acid anhydride. Thus, the sodium salt of carboxylic acid used CANNOT be ____________.
Which of the following compounds reacts with ammonia to form urotropine?
Which is the gas evolved when carboxylic acids react with strongly electropositive metals (such as Na, K, Ca, Zn)?
Which of following elements does not form amide when reacted with ammonia?
In the following reaction:
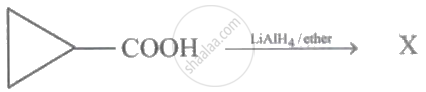
The product X is:
A flavouring agent found in oil of wintergreen is ______.
Which of the following carboxylic acids will have the highest acidity?
The elimination of CO2 from a carboxylic acid is known as ____________.
The reducing agent preferred to convert carboxylic acids and esters to primary alcohols is ____________.
Which among the following is the strongest acid?
The oxidation number of B in NaBH4 is ____________.
Which of the following aromatic acids has less acidic strength than benzoic acid?
Which of the following functional groups is reduced by diborane?
Write structure of adipic acid.
Draw structures 01 conjugate bases of monochloroacetic acid and dichloroacetic acid. Which one is more stabilized by -I effect?
How sodium bicarbonate test is used to distinguish between carboxylic acid and phenol?
