Advertisements
Advertisements
Question
Write a short note on Catenation.
Solution
Catenation: The property of carbon element due to which its atoms can join one another to form long carbon chains is called catenation.
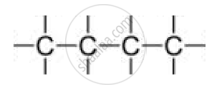
A) Straight chain of carbon atoms
B) Branched-chain of carbon atoms

C) Closed chain or ring chain of carbon atoms

APPEARS IN
RELATED QUESTIONS
Define Catenation.
Write the number of covalent bonds in the molecule of ethane.
Why does carbon form strong bonds with most other elements ?
Draw the electron dot structures for H2S.
Write the electron-dot structures for ethane.
You are given the following molecular formulae of some hydrocarbons:
C5H8; C7H14; C6H6; C5H10; C7H12; C6H12
Which two formulae represent unsaturated hydrocarbons having triple bonds?
Which of the following hydrocarbons is unsaturated?
C3H4; C2H6
Which of the two is better for our health : butter or vegetable oil? Why?
Fill in the blanks and rewrite the completed statements:
The organic compounds having double or triple bond in them are termed as _________________ _________________.
Which of the following statements are usually correct for carbon compounds? These
- are good conductors of electricity
- are poor conductors of electricity
- have strong forces of attraction between their molecules
- do not have strong forces of attraction between their molecules
Identify the unsaturated compounds from the following
- Propane
- Propene
- Propyne
- Chloropropane
Pentane has the molecular formula C5 H12 It has
Which among the following are unsaturated hydrocarbons?
- \[\ce{H3C - CH2 - CH2 - CH3}\]
- \[\ce{H3C - C ≡ C - CH3}\]
- \[\begin{array}{cc}
\ce{H3C - CH - CH3}\\
|\phantom{..}\\
\ce{CH3}\\
\end{array}\] - \[\begin{array}{cc}
\ce{H3C - C = CH2}\\
|\\
\phantom{...}\ce{CH3}\\
\end{array}\]
The name of the compound CH3 — CH2 — CHO is
Write the name of the following compound
\[\begin{array}{cc}
\phantom{....}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{O}\phantom{......}\\
\phantom{....}|\phantom{....}|\phantom{....}|\phantom{....}|\phantom{....}||\phantom{......}\\
\ce{H} - \ce{C} - \ce{C} - \ce{C} - \ce{C} - \ce{C} - \ce{OH}\\
\phantom{}|\phantom{....}|\phantom{....}|\phantom{....}|\phantom{.......}\\
\phantom{}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{.......}\\
\end{array}\]
- Write the name and draw the structure of a saturated hydrocarbon with four carbon atoms.
- Write the number of single covalent bonds present in this compound.
