Advertisements
Advertisements
Question
Write symbolic representation for the following word equation and balance them :
Carbon + Oxygen → Carbon dioxide.
Solution
Carbon + Oxygen → Carbon dioxide
`"C"+"O"_2 → "CO"_2`
APPEARS IN
RELATED QUESTIONS
Write balanced chemical equation with state symbols for the following reaction:
Sodium hydroxide solution reacts with hydrochloric acid solution to produce sodium chloride solution and water.
Write your observation for the following chemical reaction and name the product formed :
When an aqueous solution of sodium chloride is mixed with an aqueous solution of silver nitrate.
Raisins are wiped off gently before final weighing with the help of
In electrolysis of water, why is the volume of gas collected over one electrode double that of gas collected over the other electrode?
Explain the terms with examples.
Balanced equation
Write down what you understood from the following chemical reaction.
AgNO3(aq) + NaCI(aq) → AgCI ↓ + NaNO3(aq)
Write the answer to the following.
Explain the similarity and difference in two events, namely adding NaOH to water and adding CaO to water.
Write the balanced chemical equation of the following reaction.
copper + nitric acid → copper nitrate + nitric oxide + water
Write the balanced chemical equation of the following reaction.
sulphur + nitric acid→ sulphuric acid + nitrogen dioxide + water.
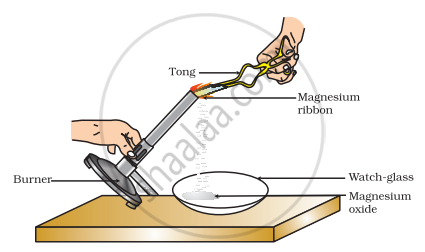
Which of the following is the correct observation of the reaction shown in the above set up?
