Advertisements
Advertisements
Question
Write the balanced chemical equation of the following reaction. sodium hydroxide + sulphuric acid → sodium sulphate + water
Solution
2NaOH + H2SO4 → Na2SO4 + 2H2O
APPEARS IN
RELATED QUESTIONS
Give one example of a chemical reaction.
Fill in the following blank with suitable word:
A solution made in water is known as an ........... solution and indicated by the symbol ...........
When hydrogen is passed over copper oxide, copper and steam are formed. Write a balanced equation for this reaction and state which of the chemicals are metals.
State one characteristic of the chemical reaction which takes place when dilute sulphuric acid is added to barium chloride solution.
Is Electrolysis of water an endothermic reaction or an exothermic reaction?
Express the following facts in the form of a balanced chemical equation:
"When a strip of copper metal is placed in a solution of silver nitrate, metallic silver is precipitated and a solution containing copper nitrate is formed".
Give one example in the case where supplying energy [given below] is necessary for a chemical reaction.
Light energy
Balance the following simple equation:
SO2 + O2 ⇌ SO3
With reference to a chemical equation state which of the statements 1 to 5 pertain to A or B.
A: Information provided by a chemical equation.
B: Limitations of a chemical equation.
- The nature of the individual elements.
- The speed of the reaction.
- The state of matter in which the substance is present.
- The completion of the reaction.
- The direction of the reaction.
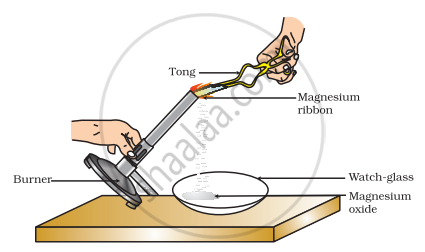
Which of the following is the correct observation of the reaction shown in the above set up?
