Advertisements
Advertisements
Question
Write the balanced chemical equation of the following reaction.
zinc sulphide + oxygen → zinc oxide + sulphur dioxide
Solution
2ZnS + 3O2 → 2ZnO + 2SO2
APPEARS IN
RELATED QUESTIONS
Write balanced chemical equation from the following information:
An aqueous calcium hydroxide solution (lime water) reacts with carbon dioxide gas to produce a solid calcium carbonate precipitate and water.
Write a balanced chemical equation for the process of photosynthesis giving the physical states of all the substances involved and the conditions of the reaction.
Balance the following chemical equation:
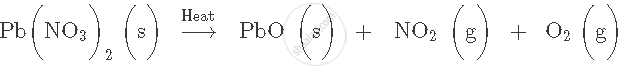
Balance the following equation. Also name the product formed.
`"Zn" + "HCI" → "ZnCI" _2 + "H"_2`
Write your observation and name the product when zinc reacts with dilute hydrochloric acid.
Balance the equation stepwise.
H2S2O7(l) + H2O(l) → H2SO4(l)
Balance the following equation:
Zn + KOH → K2ZnO2 + H2
Write the balanced chemical equation of the following reaction.
sulphur + nitric acid→ sulphuric acid + nitrogen dioxide + water.
\[\ce{MnO2 + 4HCl -> MnCl2 + 2H2O + Cl2}\]
0.02 moles of pure MnO2 is heated strongly with conc. HCl. Calculate mass of MnO2 used.
Write scientific reason.
Adding zinc particles to a solution of copper sulphate makes the blue solution colorless.
