Advertisements
Advertisements
Question
Write the balanced chemical reaction to get benzene from Sodium benzoate.
Solution
Sodium benzoate to benzene:

APPEARS IN
RELATED QUESTIONS
Identify the main product of the reaction:
\[\ce{HC ≡ C - CH3 ->[H2][Pd-C/quinoline]}\] _______.
Identify the main product of the reaction:
\[\ce{H - C ≡ C - H + H - O ->[{40%} H2SO4][{1%} HgSO4]}\] _____.
Name two reagents used for acylation of benzene.
Read the following reaction and answer the questions given below:
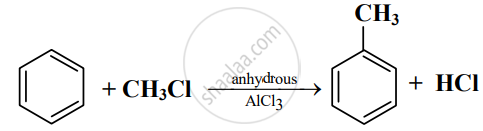
- Write the name of the reaction.
- Identify the electrophile in it.
- How is this electrophile generated?
Identify the compound (A) in the following reaction
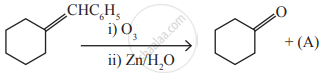
Consider the nitration of benzene using mixed con H2SO4 and HNO3 if a large quantity of KHSO4 is added to the mixture, the rate of nitration will be ______.
An alkane is obtained by decarboxylation of sodium propionate. Same alkane can be prepared by ______.
2 – butyne on chlorination gives ______.
Give IUPAC name for the following compound.
Ethyl isopropyl acetylene
Write short notes on ortho, para directors in aromatic electrophilic substitution reactions.
Suggest the route for the preparation of the following from benzene.
3 – chloro nitrobenzene
Suggest the route for the preparation of the following from benzene.
Bromo benzene
Suggest the route for the preparation of the following from benzene.
m - dinitro benzene
Write the structure of the following alkanes.
5 – (2 – Ethyl butyl) – 3, 3 – dimethyldecane
How will you prepare propane from a sodium salt of fatty acid?
Which of the following is NOT a hetero-aromatic compound?
Phenol on distillation with zinc dust gives ____________.
Direct bromination of benzene with excess reagent results in the formation of ____________.
Dow's process is used for the synthesis of an aromatic compound (X). Identify X.
Which of the following compounds on bromination yields ![]() ?
?
Read the following reaction and answer the questions given below.
\[\begin{array}{cc}
\phantom{..............................}\ce{CH3}\\
\phantom{...........................}|\\
\ce{CH3 - C = CH2 + HBr ->[benzoyl][peroxide]CH3 - CH - CH2Br}\\
|\phantom{....................................}\\
\ce{CH3}\phantom{.................................}\\
\end{array}\]
- Write the IUPAC name of the product.
- State the rule that governs the formation of this product.
Read the following reaction and answer the questions given below.
\[\begin{array}{cc}
\phantom{..............................}\ce{CH3}\\
\phantom{............................}|\\
\ce{CH3 - C = CH2 + HBr ->[benzoyl][peroxide] CH3 - CH - CH2Br}\\
|\phantom{....................................}\\
\ce{CH_3}\phantom{.................................}
\end{array}\]
- Write the IUPAC name of the product.
- State the rule that governs the formation of this product.
Read the following reaction and answer the questions given below.
\[\begin{array}{cc}
\phantom{..............................}\ce{CH3}\\
\phantom{...........................}|\\
\ce{CH3 - C = CH2 + HBr ->[benzoyl][peroxide] CH3 - CH - CH2Br}\\
|\phantom{....................................}\\
\ce{CH3}\phantom{.................................}
\end{array}\]
- Write the IUPAC name of the product.
- State the rule that governs the formation of this product.
Read the following reaction and answer the questions given below.
\[\begin{array}{cc}
\phantom{..............................}\ce{CH3}\\
\phantom{............................}|\\
\ce{CH3 - C = CH2 + HBr ->[benzoyl][peroxide]CH3 - CH - CH2Br}\\
|\phantom{....................................}\\
\ce{CH3}\phantom{.................................}
\end{array}\]
- Write IUPAC name of the product.
- State the rule that governs formation of this product.
Read the following reaction and answer the questions given below.
\[\begin{array}{cc}
\phantom{..........................}\ce{CH3}\\\phantom{........................}|\\\ce{CH3 - C = CH2 + HBr ->[benzoyl][peroxide] CH3 - CH - CH2Br}\\
|\phantom{......................................}\\
\ce{CH3}\phantom{....................................}\end{array}\]
- Write the IUPAC name of the product.
- State the rule that governs the formation of this product.
Read the following reaction and answer the questions given below.
\[\begin{array}{cc}
\phantom{..............................}\ce{CH3}\\
\phantom{............................}|\\
\ce{CH3 - C = CH2 + HBr ->[benzoyl][peroxide] CH3 - CH - CH2Br}\\
|\phantom{....................................}\\
\ce{CH3}\phantom{..................................}
\end{array}\]
- Write IUPAC name of the product.
- State the rule that governs formation of this product.
