Advertisements
Advertisements
Question
Write the reactions involved in the preparation of phenol from benzene sulfonic acid.
Solution
Preparation of phenol from benzene sulfonic acid:

RELATED QUESTIONS
Choose the correct option.
Which is the most resistant alcohol towards oxidation reaction among the following?
Answer in one sentence/ word.
Write the IUPAC name of alcohol having molecular formula C4H10O which is resistant towards oxidation.
Answer in brief.
Explain why p-nitrophenol is a stronger acid than phenol.
An ether (A), C5H12O, when heated with excess of hot HI produce two alkyl halides which on hydrolysis form compound (B) and (C), oxidation of (B) gave and acid (D), whereas oxidation of (C) gave a ketone (E). Deduce the structural formula of (A), (B), (C), (D), and (E).
Reaction between Grignard reagent and aldehyde other than formaldehyde leads to formation of _______________
The reagents used to convert phenol to 2,4,6-tribromophenol is _____________
What is the action of following on phenol at low temperature?
- dil. HNO3
- conc. H2SO4
- Br2/CS2
How will you bring about the following conversions?
acetone to 2-methylpropan-2-ol
What is the action of conc. H2SO4 on carbolic acid at 373 K.
\[\ce{C3H8O ->[KMnO4][(Oxidation)] C3H6O2}\]
The compound C3H8O is a/an ____________.
Which of the following compounds does not react with bromine in alkaline medium?
In phenols, −OH group is attached to ___________ hybridised carbon.
Propane when treated with cold cone. H2SO4 forms a compound which on heating with water gives ______.
Identify 'A' and 'B' in the following series of reactions.
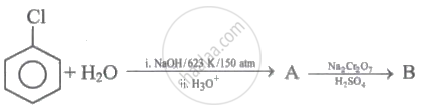
(I)
(II) \[\ce{P + Zn ->[\Delta] Q}\]
(III) \[\ce{P ->[Na2Cr2O7][H2SO4] R}\]
Q and R are respectively:
Which of the following conversion explains the acidic nature of alcohols?
Which following reagent is used to obtain alkene from alcohol?
Which of the following alcohols is least soluble in water?
Which among the following is allylic secondary alcohol?
Identify 'Z' in the following series of reaction:
\[\ce{Butan - 2 - ol ->[PCl3] X ->[alco. KOH] Y ->[i) H2SO4][ii) H-OH/heat] Z}\]
Which of the following on oxidation yields ethyl methyl ketone?
Identify the compound having highest boiling point from the following?
Identify the product X in the following reaction.
\[\ce{Phenol ->[Na2Cr2O7][H2SO4] X}\]
Which among the following phenolic compound is most acidic in nature?
Name the catalyst used in commercial method of preparation of phenol.
Which of the following compounds has lowest boiling point?
Which of the following compounds reacts immediately with Lucas reagent?
The chemical test that distinguish between benzoic acid and phenol is ______.
