Advertisements
Advertisements
Question
Write three different chemical reactions showing the conversion of ethanoic acid to sodium ethanoate. Write balanced chemical equation in each case. Write the name of the reactants and the products other ethanoic acid and sodium ethanoate in each case.
Solution
Three different chemical reactions showing the conversion of ethanoic acid to sodium ethanoate:
2CH3COOH+Na2CO3→2CH3COONa+H2O+CO2
CH3COOH+NaHCO3→CH3COONa+H2O+CO2
CH3COOH+NaOH→CH3COONa+H2O+CO2
APPEARS IN
RELATED QUESTIONS
Give balanced chemical equations for Sodium ethanoate to methane.
Complete the following equation:
`CH_3 COOH + CH_2 H_5 OH` 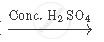
propanol into propanoic acid?
Name the process in each case and write the equations of the reactions involved.
What type to compound is CH3COOH?
On adding acetic acid to sodium hydrogen carbonate in a test tube, a student observes
(A) no reaction
(B) a colourless gas with pungent smell
(C) bubbles of a colourless and odourless gas
(D) a strong smell of vinegar
Give the structural formula of Acetic acid.
State how the following conversions can be carried out:
Ethene to Ethyl alcohol
Write the molecular formula of the given compound.
Ethanoic acid
Explain the following reaction with an example.
Esterification
Give the balanced chemical equation of the reaction.
Oxidation of ethanol by acidified potassium dichromate.
