English Medium
Academic Year: 2011-2012
Date: March 2012
Advertisements
Name the functional group present in the following compound:
CH3COCH3
Chapter:
Name the functional group present in the following compound:
C2H5CHOOH
Chapter:
Which phenomenon is responsible for making the path of light visible?
Chapter: [0.1] The Human Eye and the Colourful World
Which class of carbon compounds is responsible for the depletion of ozone layer at the higher level of the atmosphere?
Chapter: [0.13] Our Environment
Select two non-biodegradable substances from the following waste generated in a kitchen:
Spoilt food, paper bags, milk bags, vegetable peels, tin cans, used tea leaves
Chapter: [0.13] Our Environment
Define the term puberty.
Chapter: [0.07] How do Organisms Reproduce?
List two changes observed in girls at the time of puberty.
Chapter: [0.07] How do Organisms Reproduce?
What is meant by asexual reproduction?
Chapter: [0.07] How do Organisms Reproduce?
List any two different forms asexual reproduction.
Chapter: [0.07] How do Organisms Reproduce?
List four advantages of water stored in the ground.
Chapter: [0.16] Sustainable Management of Natural Resources
"Burning fossil fuels is a cause of global warming." Justify this statement.
Chapter: [0.13] Our Environment
When we place a glass prism in the path of a narrow beam of white light a spectrum is obtained. What happens when a second identical prism is placed in an inverted position with respect to the first prism? Draw a labelled ray diagram to illustrate it.
Chapter: [0.1] The Human Eye and the Colourful World
List four properties of the image formed by a convex mirror when object is placed between focus and pole of the mirror.
Chapter: [0.09] Light - Reflection and Refraction
An element 'M' has atomic number 12.
(a) Write its electronic configuration.
(b) State the group to which 'M' belongs.
(c) Is 'M' a metal or a non-metal.
(d) Write the formula of its chloride.
Chapter: [0.05] Periodic Classification of Elements
How can the valency of an element be determined if its electronic configuration is known?
Chapter: [0.05] Periodic Classification of Elements
What will be the valency of an element of atomic number 9 (nine)?
Chapter: [0.05] Periodic Classification of Elements
A star sometimes appears brighter and some other times fainter. What is this effect called? State the reason for this effect.
Chapter: [0.1] The Human Eye and the Colourful World
F, Cl and Br are the elements each having seven valence electrons. Which of these (i) has the largest atomic radius, (ii) is most reactive? Justify your answer stating reason for each.
Chapter: [0.05] Periodic Classification of Elements
List two example of each diseases caused due to (i) bacterial infection and (ii) viral infection. Which device or devices may be used to prevent the spread of such diseases.
Chapter: [0.07] How do Organisms Reproduce?
Advertisements
Distinguish between homologous organs and analogous organs. In which category would you place wings of a bird and wings of a bat? Justify your answer giving a suitable reason.
Chapter: [0.04] Carbon and its Compounds
Organic Evolution cannot be equated with progress. Explain with the help of a suitable example.
Chapter: [0.08] Heredity
A blue colour flower plant denoted by BB is crossbred with that of white colour flower plant denoted by bb.
(a) State the colour of flower you would expect in their F1 generation plants.
(b) What must be the percentage of white flower plants in F2 generation if flowers of F1 plants are self-pollinated?
(c) State the expected ratio of the genotypes BB and Bb in the F2 progeny.
Chapter: [0.07] How do Organisms Reproduce?
Complete the following equation:
CH4 + O2 —>
Chapter: [0.01] Chemical Reactions and Equations
Complete the following equation:
CH2 + H5OH `("Hot Conc." H_2SO_4)/`>
Chapter: [0.01] Chemical Reactions and Equations
Complete the following equation:
CH3COOH + NaOH →
Chapter: [0.01] Chemical Reactions and Equations
A student cannot see a chart hanging on a wall placed at a distance of 3 m from him. Name the defect of vision he is suffering from. How can it be corrected? Draw ray diagrams for the (i) defect of vision and also (ii) for its correction.
Chapter: [0.1] The Human Eye and the Colourful World
Mention the types of mirrors used as (i) rear view mirrors, (ii) shaving mirrors. List two reasons to justify your answers in each case.
Chapter: [0.09] Light - Reflection and Refraction
The image of a candle flame placed at a distance 36 cm from a spherical lens is formed on a screen placed at a distance of 72 cm from the lens. Identify the type of lens and calculate its focal length. If the height of the flame is 2.5 cm, find the height of its image.
Chapter: [0.09] Light - Reflection and Refraction
List the sign conventions for reflection of light by spherical mirrors. Draw a diagram and apply these conventions in the determination of focal length of a spherical mirror which forms a three times magnified real image of an object placed 16 cm in front of it.
Chapter:
State the law of refraction of light that defines the refractive index of a medium with respect to the other. Express it mathematically. How is refractive index of any medium 'A' with respect to a medium 'B' related to the speed of propagation of light in two media A and B? State the name of this constant when one medium is vacuum or air.
The refractive indices of glass and water with respect to vacuum are 3/2 and 4/3 respectively. If the speed of light in glass is 2 × 108m/s, find the speed of light in (i) vacuum, (ii) water.
Chapter: [0.09] Light - Reflection and Refraction
What is the difference between the chemical composition of soaps and detergents? State in brief the action of soaps in removing an oily spot from a shirt. Why are sops not considered suitable for washing where water is hard?
Chapter: [0.04] Carbon and its Compounds
List in tabular form three physical and two chemical properties on the basis of which ethanol and ethanoic acid can be differentiated.
Chapter: [0.04] Carbon and its Compounds
Answer the following question.
What is pollination? Explain the different types of pollination.
Chapter: [0.07] How do Organisms Reproduce?
Explain the term 'fertilisation'.
Chapter: [0.07] How do Organisms Reproduce?
Draw a diagram of a pistil showing pollen tube growth into the ovule and label the following:
Pollen grain, male gamete, female gamete, ovary.
Chapter: [0.07] How do Organisms Reproduce?
Advertisements
Describe in brief the role of testis in human male reproductive system.
Chapter: [0.07] How do Organisms Reproduce?
Describe in brief the role of seminal vesicle in human male reproductive system.
Chapter: [0.07] How do Organisms Reproduce?
Describe in brief the role of vas deferens in human male reproductive system.
Chapter: [0.07] How do Organisms Reproduce?
Describe in brief the role of ureter in human male reproductive system.
Chapter: [0.07] How do Organisms Reproduce?
Describe in brief the role of prostate gland in human male reproductive system.
Chapter: [0.07] How do Organisms Reproduce?
After observing the prepared slides of binary fission in Amoeba and budding in yeast following observations were reported:
(a) Single cells of Amoeba and yeast were undergoing binary fission and budding respectively.
(B) Cytokinesis was observed in the yeast cell.
(C) Elongated nucleus was dividing to form two daughter nuclei in Amoeba.
(D) A chain of buds were observed due to reproduction in Amoeba.
The correct observation(s) is/are:
(A) a and c
(B) b only
(C) c and d
(D) d, a and c
Chapter: [0.07] How do Organisms Reproduce?
A student after viewing a prepared slide illustrates the budding in yeast in the following order which is not correct:

(A) b, c, d, e, a
(B) b, e, d, c, a
(C) b, d, e, c, a
(D) b, d, c, e, a
Chapter: [0.07] How do Organisms Reproduce?
A student has to observe a permanent slide of binary fission in Amoeba. Find the correct sequence of steps given below for focussing the object under a microscope.
(a) Place the slide on the stage, look through the eye-piece and adjust the mirror to get proper illumination.
(b) Focus the slide sharp using fine adjustment screw
(c) Look through the eye-piece and raise the objective lens using coarse adjustment screw till the object is focussed.
(d) Look through the eye-piece and move the slide till the object is visible.
(A) d, c, b, a
(B) a, b, d, c
(C) a, d, c, b
(D) a, c, d, b
Chapter: [0.07] How do Organisms Reproduce?
After viewing different slides, a student draws following diagrams. Select the one which depicts binary fission in Amoeba:
 (A) a
(A) a
(B) b
(C) c
(D) d
Chapter: [0.07] How do Organisms Reproduce?
Dry raisins were soaked in water for 2 hours, to determine the percentage of water absorbed by raisins. Before final weighing of swollen raisins, the extra water left on the surface of soaked raisins was removed by:
Gently rubbing with cotton cloth
Hot air blower
Dry cotton wool
Filter paper
Chapter: [0.01] Chemical Reactions and Equations
While performing the experiment with raisins to determine the percentage of water absorbed by them, a student made following measurements:
Mass of water in the beaker = 40 g
Mass of raisins before soaking = 5 g
Mass of raisins after soaking for 2 hours = 8 g
Mass of water left in the beaker after the experiment = 35 g
The percentage of water absorbed by raisins is:
`(40g-35g)/(35g)xx100`
`(40g-35g)/(40g)xx100`
`(8g-5g)/(8g)xx100`
`(8g-5g)/(5g)xx100`
Chapter: [0.01] Chemical Reactions and Equations
Which of the following observations is true about dilute solution of acetic acid?
(A) It smells like vinegar and turns red litmus blue
(B) It smells like onion and turns blue litmus red
(C) It smells like orange and turns red litmus blue
(D) It smells like vinegar and turns blue litmus red
Chapter: [0.04] Carbon and its Compounds
A student takes Na2CO3 powder in a test tube and pours some drops of acetic acid over it. He observes:
(A) no reaction in the test tube
(B) colourless gas with pungent smell
(C) bubbles of a colourless and odourless gas
(D) white fumes with smell of vinegar
Chapter: [0.04] Carbon and its Compounds
A student adds 4 mL of acetic acid to a test tube containing 4 mL of distilled water. He then shakes the test tube and leaves it to settle. After about 10 minutes he observes:
(A) a layer of water over the layer of acetic acid
(B) a layer of acetic acid over the layer of water
(C) a precipitate settling at the bottom of the test tube
(D) a clear colourless solution
Chapter: [0.04] Carbon and its Compounds
The colours of aqueous solutions of CuSO4 and FeSO4 as observed in the laboratory are:
(A) pale green and light blue respectively
(B) light blue and dark green respectively
(C) dark blue and dark green respectively
(D) dark blue and pale green respectively
Chapter: [0.01] Chemical Reactions and Equations
A student prepared an aqueous solution of CuSO4 in beaker X and an aqueous solution of FeSO4 in beaker Y. He then dropped some iron pieces in beaker X and some zinc pieces in beaker Y. After about 10 hours he observed that the solutions in X and Y respectively appear:
(A) blue and green
(B) colourless and pale green
(C) colourless and light blue
(D) greenish and colourless
Chapter: [0.01] Chemical Reactions and Equations
While tracing the path of a ray of light passing through a rectangular glass slab a student tabulated his observations as given below:
|
S.NO. |
∠i |
∠r |
∠e |
|
I |
60° |
40° |
61° |
|
II |
50° |
36° |
51° |
|
III |
40° |
28° |
39° |
|
IV |
30° |
20° |
31° |
The correct observations is:
(A) I
(B) II
(C) III
(D) IV
Chapter: [0.09] Light - Reflection and Refraction
A student traces the path of a ray of white light through a rectangular glass slab and marks, the angles of incidence (∠i) , refraction (∠r) and emergence (∠e) as shown. Which angle or angles has he not marked correctly?
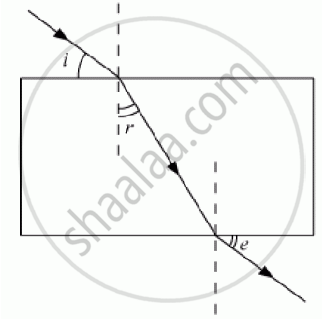
(A) ∠i only
(B) ∠i and ∠r
(C) ∠i and ∠e
(D) ∠r and ∠e
Chapter: [0.09] Light - Reflection and Refraction
To determine the focal length of a convex lens by obtaining a sharp image of a distant object we generally follow the following steps which are not in proper sequence.
(a) Hold the lens between the object and the screen
(B) Measure the distance between the lens and the screen
(C) Select a well lit distant object
(D) Place a screen opposite to the object on the lab table
(e) Adjust the position of the lens to form a sharp image
The correct sequence of these steps is:
(A) c, a, d, e, b
(B) c, d, a, e, b
(C) c, d, e, a, b
(D) c, a, e, d, b
Chapter: [0.09] Light - Reflection and Refraction
A student obtained a sharp image of the grills of a window on a screen using a concave mirror. His teacher remarked that for getting better results a well lit distant object (preferably the sun) should be focussed on the screen. What should be done for this purpose?
(A) Move the screen slightly away from the mirror
(B) Move the mirror slightly towards the screen
(C) Move the screen and the mirror away from the object
(D) Move the screen and the mirror towards the object
Chapter: [0.09] Light - Reflection and Refraction
To determine focal length of a concave mirror a student obtains the image of a well lit distant object on a screen. To determine the focal length of the given concave mirror he needs to measure the distance between:
(A) mirror and the object
(B) mirror and the screen
(C) screen and the object
(D) screen and the object and also mirror and the screen
Chapter: [0.09] Light - Reflection and Refraction
Other Solutions
Submit Question Paper
Help us maintain new question papers on Shaalaa.com, so we can continue to help studentsonly jpg, png and pdf files
CBSE previous year question papers Class 10 Science with solutions 2011 - 2012
Previous year Question paper for CBSE Class 10 Science-2012 is solved by experts. Solved question papers gives you the chance to check yourself after your mock test.
By referring the question paper Solutions for Science, you can scale your preparation level and work on your weak areas. It will also help the candidates in developing the time-management skills. Practice makes perfect, and there is no better way to practice than to attempt previous year question paper solutions of CBSE Class 10.
How CBSE Class 10 Question Paper solutions Help Students ?
• Question paper solutions for Science will helps students to prepare for exam.
• Question paper with answer will boost students confidence in exam time and also give you an idea About the important questions and topics to be prepared for the board exam.
• For finding solution of question papers no need to refer so multiple sources like textbook or guides.
