Advertisements
Advertisements
Show that the time required for 99% completion is double of the time required for the completion of 90% reaction.
Concept: Half Life Period of a Reaction
How would you account for the following:
The chemistry of actinoids is more complicated as compared to lanthanoids.
Concept: F-block Elements > The Actinoids
Give reasons for the following:
The transition metals generally form coloured compounds.
Concept: General Properties of the Transition Elements (D-block)
Define coagulation.
Concept: Properties of Colloidal Solutions
Name the method of refining of metals such as Germanium.
Concept: Refining of Crude Metals
What is the role of silica in the extraction of copper?
Concept: Occurrence of Metals
Give simple chemical tests to distinguish between the following pairs of compounds: Benzoic acid and Phenol
Concept: Chemical Properties of Phenol
How do you convert the following :
Phenol to anisole
Concept: Preparation of Ethers
Write the structures of the following molecules: XeOF4
Concept: P - Block Group 18 Elements > Concept of Group 18 Elements
Write the mechanism of the following reaction :
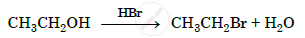
Concept: Chemical Reactions of Alcohols and Phenols > Reactions Involving Cleavage of Carbon–Oxygen (C–O) Bond in Alcohols
Why vitamin C cannot be stored in our body?
Concept: Vitamins > Classification of Vitamins
Explain the term Antiseptics
Concept: Drugs and Their Classification
Define azeotropes.
Concept: Vapour Pressure of Liquid > Vapour Pressure of Liquid- Liquid Solutions
Calculate the number of unit cells in 8.1 g of aluminium if it crystallizes in a f.c.c. structure. (Atomic mass of Al = 27 g mol–1)
Concept: Number of Atoms in a Unit Cell
Give reasons:Ferrimagnetic substances show better magnetism than antiferromagnetic substances.
Concept: Magnetic Properties
What type of azeotrope is formed by positive deviation from Raoult's law ? Give an example.
Concept: Vapour Pressure of Liquid > Vapour Pressure of Liquid- Liquid Solutions
Calculate the e.m.f. of the following cell at 298 K:
Fe(s) | Fe2+ (0.001 M) | | H+ (0.01 M) | H2(g) (1 bar) | Pt(s)
Given that \[\ce{E^0_{cell}}\] = 0.44 V
[log 2 = 0.3010, log 3 = 0.4771, log 10 = 1]
Concept: Nernst Equation - Introduction
Calculate emf of the following cell at 25°C:
\[\ce{Sn/Sn^2+ (0.001 M) || H+ (0.01 M) | H2_{(g)} (1 bar) | Pt_{(s)}}\]
Given: \[\ce{E^\circ(Sn^2+/sn) = -0.14 V, E^\circ H+/H2 = 0.00 V (log 10 = 1)}\]
Concept: Galvanic or Voltaic Cells - Introduction
Account for the following:
E° value for the Mn3+/Mn2+ couple is highly positive (+1.57 V) as compare to Cr3+/Cr2+.
Concept: Galvanic Cells - Measurement of Electrode Potential
