Advertisements
Advertisements
प्रश्न
88 cm3 of nitrogen is at a pressure of 770 mm mercury. If the pressure is raised to 880 mmHg, find by how much the volume will diminish, the temperature remaining constant.
उत्तर
V = 88 cm3
P = 770 mm
P1 = 880 mm
V1= ?
T = T1
Using gas equation,
V1 = 77 cm3
Volume diminishes = 88 - 77 = 11 cm3
APPEARS IN
संबंधित प्रश्न
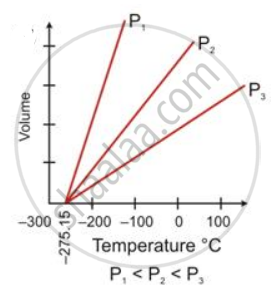
State the law which is represented by the following graph:
What will be the minimum pressure required to compress 500 dm3 of air at 1 bar to 200 dm3 temperature remaining constant?
2 liters of gas is enclosed in a vessel at a pressure of 760 mmHg. If the temperature remains constant, calculate pressure when volume changes to 4 dm3.
It is found that on heating a gas its volume increases by 50% and its pressure decreases to 60% of its original value. If the original temperature was -15°C, find the temperature to which it was heated.
Taking the volume of hydrogen as calculated in Q.19, what change must be made in Kelvin (absolute) temperature to return the volume to 2500 cm3 (pressure remaining constant)?
State Charles's law.
At a given temperature the pressure of a gas reduces to 75% of its initial value and the volume increases by 40% of its initial value. Find this temperature if the initial temperature was -10°C.
A fixed volume of a gas occupies 760cm3 at 27°C and 70 cm of Hg. What will be its vol. at s.t.p.
The pressure on one mole of gas at s.t.p. is doubled and the temperature is raised to 546 K. What is the final volume of the gas ? [one mole of a gas occupies a volume of 22.4 litres at stp.]
According to Charles’s law, at constant pressure, the temperature is inversely proportional to volume.
