Advertisements
Advertisements
प्रश्न
Balance the following reaction by oxidation number method.
\[\ce{Cr2O^2-_{7(aq)} + SO^2-_{3(aq)}->Cr^3+_{ (aq)} + SO^2-_{4(aq)}(acidic)}\]
उत्तर
\[\ce{Cr2O^2-_{7(aq)} + SO^2-_{3(aq)}->Cr^3+_{ (aq)} + SO^2-_{4(aq)}(acidic)}\]
Step 1: Write the skeletal equation and balance the elements other than O and H.
\[\ce{Cr2O^2-_{7(aq)} + SO^2-_{3(aq)}->2Cr^3+_{ (aq)} + SO^2-_{4(aq)}}\]
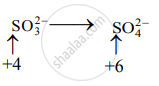
Step 2: Assign oxidation number to Cr and S. Calculate the increase and decrease in the oxidation number and make them equal.
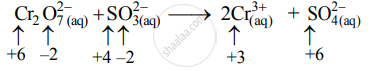
Increase in oxidation number:
(Increase per atom = 2)
Decrease in oxidation number:
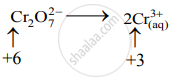
(Decrease per atom = 3)
To make the net increase and decrease equal, we must take 3 atoms of S and 2 atoms of Cr. (There are already 2Cr atoms.)
\[\ce{Cr2O^2-_{7(aq)} + 3SO^2-_{3(aq)}->2Cr^3+_{ (aq)} + 3SO^2-_{4(aq)}}\]
Step 3: Balance 'O' atoms by adding 4H2O to the right-hand side.
\[\ce{Cr2O^2-_{7(aq)} + 3SO^2-_{3(aq)}->2Cr^3+_{ (aq)} + 3SO^2-_{4(aq)} + 4H2O_{(l)}}\]
Step 4: The medium is acidic. To make the charges and hydrogen atoms on the two sides equal, add 8H+ on the left-hand side.
\[\ce{Cr2O^2-_{7(aq)} + 3SO^2-_{3(aq)} + 8H^+_{ (aq)}->2Cr^3+_{ (aq)} + 3SO^2-_{4(aq)} + 4H2O_{(l)}}\]
Step 5: Check two sides for the balance of atoms and charges.
Hence, balanced equation: \[\ce{Cr2O^2-_{7(aq)} + 3SO^2-_{3(aq)} + 8H^+_{ (aq)}->2Cr^3+_{ (aq)} + 3SO^2-_{4(aq)} + 4H2O_{(l)}}\]
APPEARS IN
संबंधित प्रश्न
Calculate the oxidation number of sulphur, chromium and nitrogen in H2SO5, `"Cr"_2"O"_7^(2-)` and `"NO"_3^-`. Suggest structure of these compounds. Count for the fallacy.
The compound AgF2 is an unstable compound. However, if formed, the compound acts as a very strong oxidizing agent. Why?
Whenever a reaction between an oxidising agent and a reducing agent is carried out, a compound of lower oxidation state is formed if the reducing agent is in excess and a compound of higher oxidation state is formed if the oxidising agent is in excess. Justify this statement giving three illustrations.
Balance the following redox reactions by ion-electron method:
- \[\ce{MnO-_4 (aq) + I– (aq) → MnO2 (s) + I2(s) (in basic medium)}\]
- \[\ce{MnO-_4 (aq) + SO2 (g) → Mn^{2+} (aq) + HSO-_4 (aq) (in acidic solution)}\]
- \[\ce{H2O2 (aq) + Fe^{2+} (aq) → Fe^{3+} (aq) + H2O (l) (in acidic solution)}\]
- \[\ce{Cr_2O^{2-}_7 + SO2(g) → Cr^{3+} (aq) + SO^{2-}_4 (aq) (in acidic solution)}\]
The Mn3+ ion is unstable in solution and undergoes disproportionation to give Mn2+, MnO2, and H+ ion. Write a balanced ionic equation for the reaction.
In Ostwald’s process for the manufacture of nitric acid, the first step involves the oxidation of ammonia gas by oxygen gas to give nitric oxide gas and steam. What is the maximum weight of nitric oxide that can be obtained starting only with 10.00 g. of ammonia and 20.00 g of oxygen?
Justify that the following reaction is redox reaction; identify the species oxidized/reduced, which acts as an oxidant and which acts as a reductant.
\[\ce{2Cu2O_{(S)} + Cu2S_{(S)}->6Cu_{(S)} + SO2_{(g)}}\]
Balance the following reaction by oxidation number method.
\[\ce{MnO^-_{4(aq)} + Br^-_{ (aq)}->MnO2_{ (s)} + BrO^-_{3(aq)}(basic)}\]
Balance the following reaction by oxidation number method.
\[\ce{H2SO4_{(aq)} + C_{(s)}->CO2_{(g)} + SO2_{(g)} + H2O_{(l)}(acidic)}\]
Balance the following reaction by oxidation number method.
\[\ce{Bi(OH)_{3(s)} + Sn(OH)^-_{3(aq)}->Bi_{(s)} + Sn(OH)^2-_{6(aq)}(basic)}\]
Balance the following redox equation by half-reaction method.
\[\ce{Bi(OH)_{3(s)} + SnO^2-_{2(aq)}->SnO^2-_{3(aq)} + Bi^_{(s)}(basic)}\]
Which of the following is INCORRECT for the following reaction?
\[\ce{2Zn_{(s)} + O2_{(g)} -> 2ZnO_{(s)}}\]
Which of the following is a redox reaction?
Identify the oxidising agent in the following reaction:
\[\ce{CH4_{(g)} + 2O2_{(g)} -> CO2_{(g)} + 2H2O_{(l)}}\]
Write balanced chemical equation for the following reactions:
Reaction of liquid hydrazine \[\ce{(N2H4)}\] with chlorate ion \[\ce{(ClO^{-}3)}\] in basic medium produces nitric oxide gas and chloride ion in gaseous state.
Write balanced chemical equation for the following reactions:
Dichlorine heptaoxide \[\ce{(Cl2O7)}\] in gaseous state combines with an aqueous solution of hydrogen peroxide in acidic medium to give chlorite ion \[\ce{(ClO^{-}2)}\] and oxygen gas. (Balance by ion-electron method)
Balance the following equations by the oxidation number method.
\[\ce{I2 + NO^{-}3 -> NO2 + IO^{-}3}\]
Balance the following equations by the oxidation number method.
\[\ce{I2 + S2O^{2-}3 -> I- + S4O^{2-}6}\]
Identify the redox reactions out of the following reactions and identify the oxidising and reducing agents in them.
\[\ce{3HCl (aq) + HNO3 (aq) -> Cl2 (g) + NOCl (g) + 2H2O (l)}\]
Balance the following ionic equations.
\[\ce{Cr2O^{2-}7 + H^{+} + I- -> Cr^{3+} + I2 + H2O}\]
Balance the following ionic equations.
\[\ce{Cr2O^{2-}7 + Fe^{2+} + H+ -> Cr^{3+} + Fe^{3+} + H2O}\]
Balance the following ionic equations.
\[\ce{MnO^{-}4 + SO^{2-}3 + H^{+} -> Mn^{2+} + SO^{2-}4 + H2O}\]
Balance the following ionic equations.
\[\ce{MnO^{-}4 + H^{+} + Br^{-} -> Mn^{2+} + Br2 + H2O}\]
In acidic medium, reaction, \[\ce{MNO^-_4 → Mn^2+}\] an example of ____________.
In \[\ce{Cu^{2+} + Ag -> Cu + Ag^+}\], oxidation half-reaction is:
\[\ce{H2O2 -> 2H^+ + O2 + 2e^-}\]; E0 = −0.68 V.
This equation represents which of the following behaviour of H2O2?
