Advertisements
Advertisements
प्रश्न
Bronze is an alloy of ______.
विकल्प
copper and tin
copper and zinc
tin and zinc
copper, zinc and tin
उत्तर
Bronze is an alloy of copper and tin.
Explanation:
Bronze is a solid alloy composed primarily of 88% copper and 12% tin. It is used in the production of hardware, utensils musical instruments, medals, and other items. Due to its resistance to corrosion caused by seawater, it is used in submerged bearings and ship propellants.
APPEARS IN
संबंधित प्रश्न
Write two methods of preventing the rusting of iron.
Answer the following question.
What are alloys?
Explain why rusting of iron objects is faster in coastal areas than in deserts.
Choose the correct answer from the options given below:
Heating an ore in a limited supply of air or in the absence of air at a temperature just below its melting point is known as
A. Smelting
B. Ore-dressing
C. Calcination
D. Bessemerisation
What special name is given to their corrosion of iron?
What is meant by galvanisation? Why is it done?
State two conditions for the rusting of iron.
In one method of rust prevention, the iron is not coated with anything. Which is this method?
Fill in the following blank with suitable word:
The corrosion of iron is called ................
Fill in the following blanks with suitable words:
Tiffin boxes are electroplated with .............. but car bumpers are electroplated with ............... to protect them from rusting.
Fill in the following blanks with suitable words:
............ and .............. are necessary for the rusting of iron.
Name any two metals which do not corrode easily.
Give the constituents and one use of brass.
Name two metals which resist corrosion due to the formation of a thin, hard and impervious layer of oxide on their surface.
What are the constituents of stainless steel?
Explain why, when a copper object remains in damp air for a considerable time, a green coating is formed on its surface. What is this process known as?
Name a common metal which is highly resistant to corrosion.
If copper is kept exposed to damp air for a considerable time, it gets a green coating on its surface. This is due to the formation of:
(a) hydrated copper sulphate
(b) copper oxide
(c) basic copper carbonate
(d) copper nitrate
Explain how the activity series accounts for each of the following:
tendency to corrosion
What is corrosion? What are necessary conditions for corrosion?
Corrosion can be an advantage in some case.Explain ?
Observe the following picture a write down the chemical reaction with the explanation.

Identify the process shown in the diagram and explain it in short
Find the odd man out:
What is done to prevent corrosion of metals?
Answer the following question:
What is corrosion? Do gold ornaments corrode? Justify.
Write three methods of preventing rusting of iron.
What is "rusting"? Describe with a labelled diagram an activity to investigate the conditions under which iron rusts.
Give a reason why rust turns moist red litmus blue.
State whether the statement given below is true or false. If false write the correct statement.
Either oxygen or moisture is essential for rusting.
State whether the statement given below is true or false. If false write the correct statement.
Graphite is a lustrous non-metal which conducts electricity.
_______ is an alloy made from iron, carbon and chromium.
Which of the following method is used to prevent the accumulation of greenish layer on brass due to corrosion?
_______ forms a green colour in the water.
Find the odd one out and give its explanation.
Find the odd one out and give its explanation.
Write the name.
Method used to prevent corrosion of copper.
Write scientific reason.
On exposure to air, silver articles turn blackish after some time.
Write scientific reason.
Coins are made from metals and alloys.
Explain concept with example/explain with the help of a balanced equation.
Corrosion
Draw a neat labelled diagram.
Electroplating
Complete flow chart given below.

Write a molecular formula for rust.
Observe the following diagram and give answers.
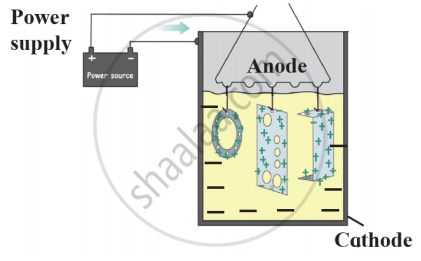
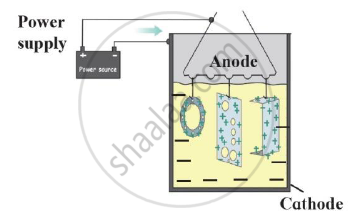
- Name this method of prevention of corrosion.
- For prevention of which metal this method is used?
- What is used as anode in this method?
The process of coating the surface of the metal with a thin layer of zinc is called ______
What is rust?
Amalgam is an alloy of ____________.
Which among the following alloys contain mercury as one of its constituents?
The diagram shows the reaction between metal and dil. acid.
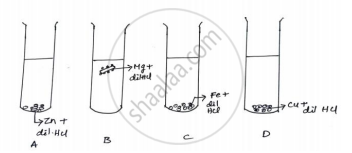
What is the reason for different behaviour of Mg in test tube B?
The table shown below gives information about four substances: A, B, C and D.
| SUBSTANCE | MELTING POINT (K) | ELECTRICAL CONDUCTIVITY | |
| SOLID | LIQUID/ AQUEOUS | ||
| A | 295 | Good | Good |
| B | 1210 | Poor | Good |
| C | 1890 | Poor | Good |
| D | 1160 | Poor | Poor |
Identify Ionic compounds from the above given substances.
Marble’s popularity began in ancient Rome and Greece, where white and off-white marble were used to construct a variety of structures, from hand-held sculptures to massive pillars and buildings.

The substance not likely to contain CaCO3 is:
Identify the correct statement from the following:
Alloys are homogeneous mixtures of a metal with a metal or nonmetal. Which among the following alloys contain non-metal as one of its constituents?
Generally, when metals are treated with mineral acids, hydrogen gas is liberated but when metals (except Mn and Mg), treated with HNO3, hydrogen is not liberated, why?
Explain the following:
Lime water turns milky on passing carbon dioxide gas into it.
Give an example of a chemical reaction for each of the following situations:
Sound is produced
In ______ process a layer of molten tin is deposited on metals.
