Advertisements
Advertisements
प्रश्न
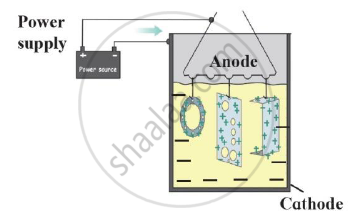
Draw a neat labelled diagram.
Electroplating
उत्तर
Electroplating-

APPEARS IN
संबंधित प्रश्न
Explain the terms Corrosion
Explain why rusting of iron objects is faster in coastal areas than in deserts.
Name any three objects (or structures) which are gradually damaged by the corrosion of iron and steel.
The chemical reaction involved in the corrosion of iron metal is that of:
(a) oxidation as well as displacement
(b) reduction as well as combination
(c) oxidation as well as combination
(d) reduction as well as displacement
Name the metal which is used for galvanising iron.
Explain why, iron sheets are coated with zinc.
Fill in the following blank with suitable word:
The corrosion of copper produces a .............. coating of basic copper carbonate on its surface
Name any two metals which do not corrode easily.
What is meant by 'rusting of iron'? With the help of labelled diagrams, describe an activity to find out the conditions under which iron rusts.
Name a common metal which is highly resistant to corrosion.
A common metal which is highly resistant to corrosion is:
(a) iron
(b) copper
(c) aluminium
(d) magnesium
In stainless steel alloy, iron metal is mixed with:
(a) Cu and Cr
(b) Cr and Ni
(c) Cr and Sn
(d) Cu and Ni
Explain how the activity series accounts for each of the following:
tendency to corrosion
State under what conditions corrosion is faster ?
Compare roasting and calcination.
Observe the following picture a write down the chemical reaction with the explanation.

Identify the process shown in the diagram and explain it in short
Find the odd man out:
What is done to prevent corrosion of metals?
Give reason.
A wooden article should be polished.
State whether the statement given below is true or false. If false write the correct statement.
Graphite is a lustrous non-metal which conducts electricity.
_______ is an alloy made from iron, carbon and chromium.
Find the odd one out and give its explanation.
Write the name.
An alloy of copper and tin-
Write scientific reason.
On exposure to air, silver articles turn blackish after some time.
Observe the following diagram and write answers.

- Name the method.
- Explain the method.
- Give two examples of this method.
Give preventive methods by giving examples of corrosion?
The process of coating the surface of the metal with a thin layer of zinc is called ______
State two conditions necessary for rusting of iron.
The table shown below gives information about four substances: A, B, C and D.
| SUBSTANCE | MELTING POINT (K) | ELECTRICAL CONDUCTIVITY | |
| SOLID | LIQUID/ AQUEOUS | ||
| A | 295 | Good | Good |
| B | 1210 | Poor | Good |
| C | 1890 | Poor | Good |
| D | 1160 | Poor | Poor |
Identify Ionic compounds from the above given substances.
Generally, when metals are treated with mineral acids, hydrogen gas is liberated but when metals (except Mn and Mg), treated with HNO3, hydrogen is not liberated, why?
Explain the following:
Bubbles are produced when acetic acid is added to a solution of sodium hydrogencarbonate.
Gold plated ornaments is the example of ______.
Explain the chemical reactions in rusting of iron.
