Advertisements
Advertisements
प्रश्न
Draw a neat labelled diagram.
Electroplating
उत्तर
Electroplating-

APPEARS IN
संबंधित प्रश्न
Why do we apply paint on iron articles?
Two methods by which rusting of iron can be prevented are ______ and ______.
Fill in the following blank with suitable word:
The corrosion of copper produces a .............. coating of basic copper carbonate on its surface
Name any two metals which do not corrode easily.
What is meant by 'rusting of iron'? With the help of labelled diagrams, describe an activity to find out the conditions under which iron rusts.
Give the constituents and one use of brass.
Explain why, when a copper object remains in damp air for a considerable time, a green coating is formed on its surface. What is this process known as?
A common metal which is highly resistant to corrosion is:
(a) iron
(b) copper
(c) aluminium
(d) magnesium
Mention two uses of the following metals and non-metals
Iron
Bronze is an alloy of ______.
State under what conditions corrosion is faster ?
Explain with reason:
Roasting is carried out on sulphide ores and not on carbonate ores.
No chemical reaction takes place when granules of a solid, A, are mixed with the powder of another solid, B. However when the mixture is heated, a reaction takes place between its components. One of the products, C, is a metal and settles down in the molten state while the other product, D, floats over it. It was observed that the reaction is highly exothermic.
(i) Based on the given information make an assumption about A and B and write a chemical equation for the chemical reaction indicating the conditions of reaction, physical state of reactants and products and thermal status of reaction.
(ii) Mention any two types of reactions under which above chemical reaction can be classified.
Complete the process of iron rusting by filling the blanks. Suggest a way to prohibit the process.
The iron rust is formed due to........................... reaction. Different
regions on iron surface become anode and cathode.
Reaction on anode region :
`F_e(s) → Fe^(2+) (aq) +2e^-`
Reaction on anode region :
`O_2(g) + 4H^+(aq) +............................ → 2H_2 O (l) `
When Fe2+ ions migrate from anode region they react with ................... to form Fe3+ ions.
A reddish coloured hydrated oxide is formed from ............... ions. It is called rust.
`2Fe_(3+) (aq) + 4H_2O(l) → ................. + 6H_+(aq) `
A way to prevent rusting ..................................................................
Find the odd man out:
Choose the correct alternative and rewrite the following:
Iron is _____________________.
Give reason.
Copper and brass utensils should be tinned.
Corrosion of silver causes a black layer of _______.
When one of the metals in an alloy is mercury the alloy is called _______.
Find the odd one out and give its explanation.
Write scientific reason.
Anodization method is useful for prevention of the corrosion of the aluminium.
Write scientific reason.
Coins are made from metals and alloys.
Explain concept with example/explain with the help of a balanced equation.
Corrosion
What is rust?
Write a molecular formula for rust.
Observe the following diagram and give answers.
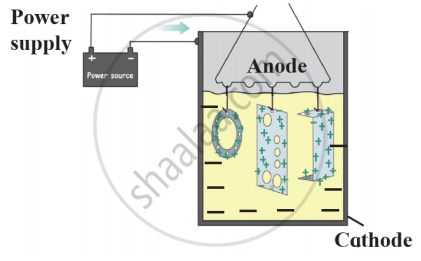
- Name this method of prevention of corrosion.
- For prevention of which metal this method is used?
- What is used as anode in this method?
Observe the following diagram and write answers.

- Name the method.
- Explain the method.
- Give two examples of this method.
The process of coating the surface of the metal with a thin layer of zinc is called ______
What is rust?
Give the equation for the formation of rust.
Copper objects lose their shine and form green coating of ____________.
Which among the following alloys contain mercury as one of its constituents?
Identify the correct statement from the following:
Alloys are homogeneous mixtures of a metal with a metal or nonmetal. Which among the following alloys contain non-metal as one of its constituents?
State whether the following statements are true or false:
Ships suffer a lot of damage though they are painted.
Gold plated ornaments is the example of ______.
| A process of forming a thick oxide of aluminium when aluminium is exposed to air. This coat makes it resistant to corrosion. Resistance can be improved by making a layer of oxide thinker. In this technique, the aluminium article is the anode, and the electrolyte is sulphuric acid. The anode reaction results in the formation of a black-coloured film of aluminium oxide on the anode. By putting appropriate dyes in the electrolytic solution, both coloured surface with the decorative finish is achieved. Kitchen articles like anodised such as pressure cookers, pans and frames of sliding windows are applications of this technique. |
- Name the anode and electrolyte used in this technique.
- How can we make aluminium articles made resistant to corrosion?
- Name the technique used to coat the aluminium articles.
In ______ process a layer of molten tin is deposited on metals.
