Advertisements
Advertisements
प्रश्न
Calculate the binding energy of an alpha particle in MeV. Given
mass of a proton = 1.007825 u
mass of a neutron = 1.008665 u
mass of He nucleus = 4.002800 u
1u = 931 MeV/c2
उत्तर
Given:
mp = 1.007825 u
mn = 1.008665 u
mα = 4.002800 u
B.E. = Δmc2
= `{(2m_p + 2m_n) - m_alpha}c^2`
= `{(2 xx 1.007825 + 2 xx 1.008665) - 4.002800} xx c^2`
B.E. = `0.03018 xx 931` MeV
B.E. = 28.09758 MeV
APPEARS IN
संबंधित प्रश्न
Obtain the binding energy (in MeV) of a nitrogen nucleus `(""_7^14"N")`, given `"m"(""_7^14"N")` = 14.00307 u.
Use this graph to explain the release of energy in both the processes of nuclear fusion and fission.
What is the minimum energy which a gamma-ray photon must possess in order to produce electron-positron pair?
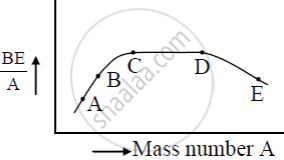
The figure shows the plot of binding energy (BE) per nucleon as a function of mass number A. The letters A, B, C, D, and E represent the positions of typical nuclei on the curve. Point out, giving reasons, the two processes (in terms of A, B, C, D, and E ), one of which can occur due to nuclear fission and the other due to nuclear fusion.
In a periodic table the average atomic mass of magnesium is given as 24.312 u. The average value is based on their relative natural abundance on earth. The three isotopes and their masses are\[\ce{_12^24Mg}\](23.98504 u), \[\ce{_12^25Mg}\] (24.98584 u), and \[\ce{_12^26Mg}\] (25.98259 u). The natural abundance of \[\ce{_12^24Mg}\] is 78.99% by mass. Calculate the abundances of other two isotopes.
The difference in mass of a nucleus and its constituents is called ______.
A body's centre of mass
He23 and He13 nuclei have the same mass number. Do they have the same binding energy?
Find the binding energy of a H-atom in the state n = 2
State the significance of binding energy per nucleon.
