Advertisements
Advertisements
प्रश्न
Define σ – bond.
उत्तर
When two atomic orbitals overlap linearly along the axis, the resultant bond is called a sigma (σ) bond.
APPEARS IN
संबंधित प्रश्न
Draw diagram for bonding in ethene with sp2 Hybridisation.
Display electron distribution around the oxygen atom in the water molecule and state the shape of the molecule, also write the H-O-H bond angle.
Give reasons for need of Hybridization
Explain geometry of methane molecule on the basis of Hybridization.
Identify the type of overlap present in H-F molecule. Explain diagrammatically.
Complete the following Table.
| Molecule | Type of Hybridization | Type of bonds | Geometry | Bond angle |
| CH4 | - | 4C-H 4σ bonds |
Tetrahedral | - |
| NH3 | sp3 | 3N-H 3σ bonds 1 lone pair |
- | - |
| H2O | - | - | angular | 104.5° |
| BF3 | sp2 | - | - | 120° |
| C2H4 | - | - | - | 120° |
| BeF2 | - | 2 Be-F | Linear | - |
| C2H2 | sp | (3σ+2π) 1C-C σ 2C-H σ 2C-C π |
- | - |
Give the type of overlap by which the pi (π) bond is formed.
Mention the steps involved in Hybridization.
The number of sigma bonds in vanillin is ____________.
Why does type of overlap given in the following figure not result in bond formation?
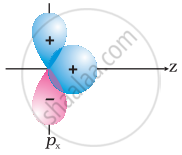 |
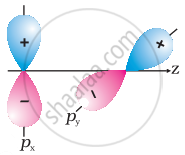 |
