Advertisements
Advertisements
प्रश्न
Explain VSEPR theory. Applying this theory to predict the shapes of IF7 and SF6.
उत्तर
Lewis’s concept of the structure of molecules deals with the relative position of atoms in the molecules and sharing of electron pairs between them. However, we cannot predict the shape of the molecule using Lewis concept. Lewis theory in combination with VSEPR theory will be useful in predicting the shape of molecules.
IF7:
Iodine has 7 valence electrons in the valence shell. In an excited state it has 6 valency electrons by 1st and 2nd excited state & under sp3d2, hybridization and combines with 7 Fluorine to get pentagonal bipyramidal shape. There are no lone pairs.
I = ns2 np5 nd0


pentagonal bipyramidal
SF6:
Sulphar attain six valence electron in 1st and 2nd excited states undergoes sp3d2 hybridization and combines with six ‘F’ atoms, as there no lone pair electrons it geometry in octahedral.
S = 16 = ns2 np4 nd0

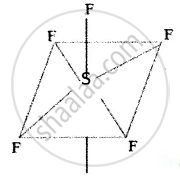
Octahedral
APPEARS IN
संबंधित प्रश्न
Although geometries of NH3 and H2O molecules are distorted tetrahedral, bond angle in water is less than that of ammonia. Discuss.
Select and write the most appropriate alternatives from the given choices.
Valence Shell Electron Pair repulsion (VSEPR) theory is used to predict which of the following:
Shape of ClF3 is ______.
The H-N-H bond angle in NH3 molecule is ____________.
Stable form of A may be represented by the formula:
Which of the possible molecule/species is having maximum values for dipole moment. (where "A" is the central atom)?
Consider the species CH4, `"NH"_4^+` and `"BH"_4^-`. Choose the correct option with respect to these species.
The number of lone pairs of electrons on the central I atom in `"I"_3^-` is ______.
In the following structure, the percentage of the 's' character in the lone pair occupy by the oxygen atom is ______.
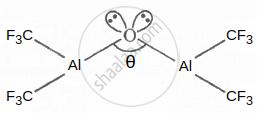
Given: Cos θ = −0.99
What is the number of lone pair of electrons in IF7?
