Advertisements
Advertisements
प्रश्न
Select and write the most appropriate alternatives from the given choices.
Valence Shell Electron Pair repulsion (VSEPR) theory is used to predict which of the following:
विकल्प
Energy levels in an atom
the shapes of molecules and ions
the electrone getivities of elements
the type of bonding in compounds
उत्तर
Valence Shell Electron Pair repulsion (VSEPR) theory is used to predict which of the following: the shapes of molecules and ions
APPEARS IN
संबंधित प्रश्न
Discuss the shape of the following molecules using the VSEPR model:
BeCl2, BCl3, SiCl4, AsF5, H2S, PH3
Although geometries of NH3 and H2O molecules are distorted tetrahedral, bond angle in water is less than that of ammonia. Discuss.
Shape of ClF3 is ______.
Explain VSEPR theory. Applying this theory to predict the shapes of IF7 and SF6.
The H-N-H bond angle in NH3 molecule is ____________.
Select the INCORRECT match.
Identify the molecule with linear geometry?
Stable form of A may be represented by the formula:
Explain the non-linear shape of \[\ce{H2S}\] and non-planar shape of \[\ce{PCl3}\] using valence shell electron pair repulsion theory.
Elements \[\ce{X, Y}\] and \[\ce{Z}\] have 4, 5 and 7 valence electrons respectively. Write the molecular formula of the compounds formed by these elements individually with hydrogen.
Elements \[\ce{X, Y}\] and \[\ce{Z}\] have 4, 5 and 7 valence electrons respectively. Which of these compounds will have the highest dipole moment?
Consider the species CH4, `"NH"_4^+` and `"BH"_4^-`. Choose the correct option with respect to these species.
Number of lone pair(s) of electrons on central atom and the shape of BrF3 molecule respectively are ______.
The number of lone pairs of electrons on the central I atom in `"I"_3^-` is ______.
Given below are two statements: One is labelled as Assertion A and the other is labelled as Reason R:
Assertion A: The H-O-H bond angle in water molecule is 104.5°.
Reason R: The lone pair-lone pair repulsion of electrons is higher than the bond pair-bond pair repulsion.
In the light of the above statements, choose the correct answer from the options given below:
In the following structure, the percentage of the 's' character in the lone pair occupy by the oxygen atom is ______.
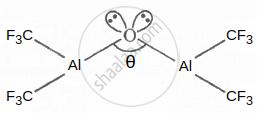
Given: Cos θ = −0.99
What is the geometry of a water molecule?
What is the number of lone pair of electrons in IF7?
Identify compound having square pyramidal-shape:from following.
